File
advertisement
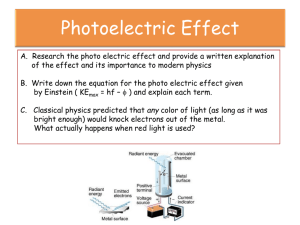
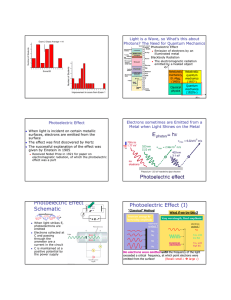
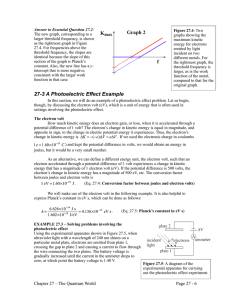
PHOTOELECTRIC EFFECT QUICK REVIEW • DUAL NATURE OF LIGHT: Light is an electromagnetic wave that has both wave and particle properties. • QUANTUM THEORY: Light consists of bundles of energy called photons. The energy is directly proportional to the frequency of the light. ENERGY OF A PHOTON: E=hf or E=hc λ E = photon energy ( J or eV) C =speed of light (3.0x108m/s) h = Planck’s constant (h= 6.63x10-34J·s) f = frequency (Hz) λ = wavelength (m) EXAMPLE Ex: A photon of red light has a frequency of 4.22x1014Hz. How much energy does it have in joules? In electron volts? E = hf E = 6.63x10-34J·s(4.22x1014Hz) E = 2.80x10-19J= 2.80x10-19J 1.6x10-19J/eV E= 1.75eV Energy vs. Frequency Energy vs. wavelength E(J) E(J) f(Hz) λ(m) Important terms • Photoelectric effect- The emission of an electron from a metal plate due to exposure to electromagnetic waves of a certain frequency. • Threshold Frequency (fo)- minimum frequency needed to move photoelectrons. Varies with each metal. If the frequency is too low, no electrons are emitted. If the frequency is equal to the threshold frequency, the electron is emitted but doesn’t move. If the frequency is greater than the threshold frequency, the electron is emitted from the plate and increases in velocity. Kinetic energy of photoelectrons Threshold frequency is different for each metal. As the frequency increases, the kinetic energy of the emitted electron increases. The slope of every graph is equal to Planck’s constant. KE(J) Fo (Hz) NOTE: KE = 1/2mv2 If velocity increases so does the kinetic energy LIGHT INTENSITY Increasing the intensity of the light (brightness) will increase the number of released electrons but it won’t increase the kinetic energy of the electrons. KE(J) INTENSITY Solar Cells: Sunlight hits a metal plate and releases electrons. This causes a flow of electrons which produces electrical energy. silicon, cadmium telluride, and copper indium selenide-sulfide.