Cambridge Physics for the IB Diploma
Mark scheme for Support Worksheet – Topic 6,
Worksheet 3
1
a
b
2
a
b
3
a
b
4
5
The energy carried by a wave is distributed along wavefronts; if we consider a
very low intensity beam of light incident on the metal the energy is being
delivered to the metal at a very low rate and so the time required for the
electron to accumulate the energy needed to escape would be very large.
[2]
According to the photon theory of light, the energy of light is carried by
individual photons; and the entire energy carried by the photon is transferred to
the electron at once when the photon is absorbed.
[2]
(Whereas the energy carried by mechanical waves depends on both frequency
and amplitude) the energy carried by electromagnetic waves depends only on
the amplitude and not the frequency; hence the energy of the emitted electron
cannot have any dependence on frequency.
[2]
According to the photon theory the energy of a photon is given by hf ; hence
the energy of the emitted electron is hf and so depends on the light
frequency.
[2]
The intensity has no effect on the energy of the electrons; increased intensity
implies more photons not more energetic photons.
[2]
More electrons would be ejected; increased intensity implies more photons and
hence greater probability for more electrons to be ejected from the metal.
[2]
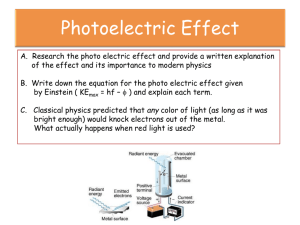
The work function is the minimum energy required to eject an electron from a metal
surface.
EK hf
1.00 10
hc
19
[1]
6.63 10 34 3 108
2.2 1.6 10 19
4.2 10 7
J
1 2
2.00 10 19
mv 1.00 10 19 J v
4.7 10 5 m s 1
31
2
9.1 10
6
a
b
7
a
b
[3]
Stopping voltage is that voltage between the anode and the cathode in a
photoelectric effect experiment for which the photocurrent becomes zero.
From eVS hf VS
[1]
hf
h
the slope is , i.e. Planck’s constant divided
e e
e
by the elementary charge.
[1]
Particles show wavelike behaviour; with a wavelength that equals Plank’s
constant divided by the particle’s momentum.
[2]
See Physics for the IB Diploma, pages 394–395.
[3]
Copyright Cambridge University Press 2012. All rights reserved.
Page 1 of 2
Cambridge Physics for the IB Diploma
8
9
10
p2
150 1.6 10 19 p 6.6110 24 Ns; and so
2m
h 6.63 10 34
1.0 10 10 m
24
p 6.61 10
2L
with n 1,2,3
n
a
The allowed wavelengths are
b
E
a
The product of the uncertainties in momentum and position is never less than
Planck’s constant divided by 4π .
b
p2
h2
; and so E
2m 2m 2
h2
h 2 n2
2
2L
2m( )2 8mL
n
Since x : 1010 m (this is an order of magnitude calculation so you may
1
choose x : 10 10 m if you wish);
2
h
6.63 1034
p
5.3 1025 1024 Ns
10
4πx 4π 10
Copyright Cambridge University Press 2012. All rights reserved.
[2]
[1]
[2]
[1]
[2]
Page 2 of 2