HW 5 (Ch. 6) Quiz Question 1
advertisement

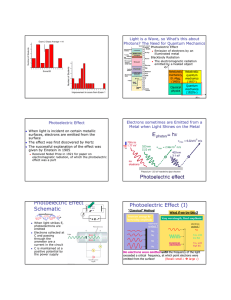
HW 5 (Ch. 6) Quiz Question 1 HW 5 (Ch. 6) Quiz Question 2 A metal surface ejects electrons when illuminated with green light but not with yellow light. Which of the following colors will not eject electrons from the metal? A. Blue B. Violet C. Red Before digital photography people worked in “dark” rooms to print photos. The rooms usually had a red light so they were not totally dark. Why red and not green or blue illumination? A. Red light has lower energy photons than can effect most photographic paper. B. Red light is easier on he eyes C. Green and blue light bulbs are more expensive than red. D. The eye is more sensitive to red light so the room may be illuminated at a lower intensity. Discovery: Heinrich Hertz and Phillip Lenard HW 5 (Ch. 6) Quiz Question 3 You all have experience with a good mirror. Think about what you see in a mirror. Now consider that about 80% of the light incident on the mirror is reflected. How do you know 20% of the incident photons are absorbed, and not that 20% of the energy of each photon is lost? A. We don’t – it would take more measurements. B. The color of the light does not change Hertz’s Spark Gap Generator: Back in 1887… Hertz clarified Maxwell’s electromagnetic theory of light: Proved that electricity can be transmitted in electromagnetic waves. Established that light was a form of electromagnetic radiation. First person to broadcast and receive these waves. Lenard Goes Further… His assistant, Phillip Lenard, explored the effect further. He built his own apparatus called a “phototube” to determine the nature of the effect: 1 Light is a Wave, so What’s this about Photons? The Need for Quantum Mechanics Lenard’s Photoelectric Apparatus: Photoelectric Effect Emission of electrons by an illuminated metal v/c Relativistic mechanics, El.-Mag. (1905) Relativistic quantum mechanics (1927-) Classical physics Quantum mechanics (1920’s-) h /s Electrons sometimes are Emitted from a Metal when Light Shines on the Metal Photoelectric Effect When light is incident on certain metallic surfaces, electrons are emitted from the surface The effect was first discovered by Hertz The successful explanation of the effect was given by Einstein in 1905 Received Nobel Prize in 1921 for paper on electromagnetic radiation, of which the photoelectric effect was a part Photoelectric Effect Schematic Photoelectric Effect-What’s Going On? I e+V _ When light strikes E, photoelectrons are emitted Electrons collected at C and passing through the ammeter are a current in the circuit C is maintained at a positive potential by the power supply “Classical” Method Increase energy by increasing amplitude electrons emitted ? No No No No What if we try this ? Vary wavelength, fixed amplitude electrons emitted ? No Yes, with low KE Yes, with high KE No electrons were emitted until the frequency of the light exceeded a critical frequency, at which point electrons were emitted from the surface! (Recall: small large ) 2 Photoelectric Effect-Classical Physics Enter a real Einstein Electrons are attracted to the (positively charged) nucleus by the electrical force In metals, the outermost electrons are not tightly bound, and can be easily “liberated” from the shackles of its atom. An alternate view is that light is acting like a particle—one light particle is needed to eject one electron It just takes sufficient energy… The light particle must have sufficient energy to “free” the electron from the atom. Classically, we increase the energy of an EM wave by increasing the intensity (e.g. brightness) Energy A2 But this doesn’t work ?? Increasing the Amplitude is simply increasing the number of light particles, and two light particles can’t add to one electron’s energy at the same time! However, if the energy of these “light particle” is related to their frequency, this would explain why higher frequency light can knock the electrons out of their atoms, but low frequency light cannot… Quantum Mechanics In this “quantum-mechanical” picture, the energy of the light particle (photon) must overcome the binding energy of the electron to the nucleus. If the energy of the photon exceeds the binding energy, the electron is emitted with a KE = Ephoton – Ebinding. The energy of the photon is given by E=hwhere the constant h = 6.6x10-34 [J s] is Planck’s constant. “Light particle” Before Collision After Collision 3