Modern Physics Work Package
advertisement
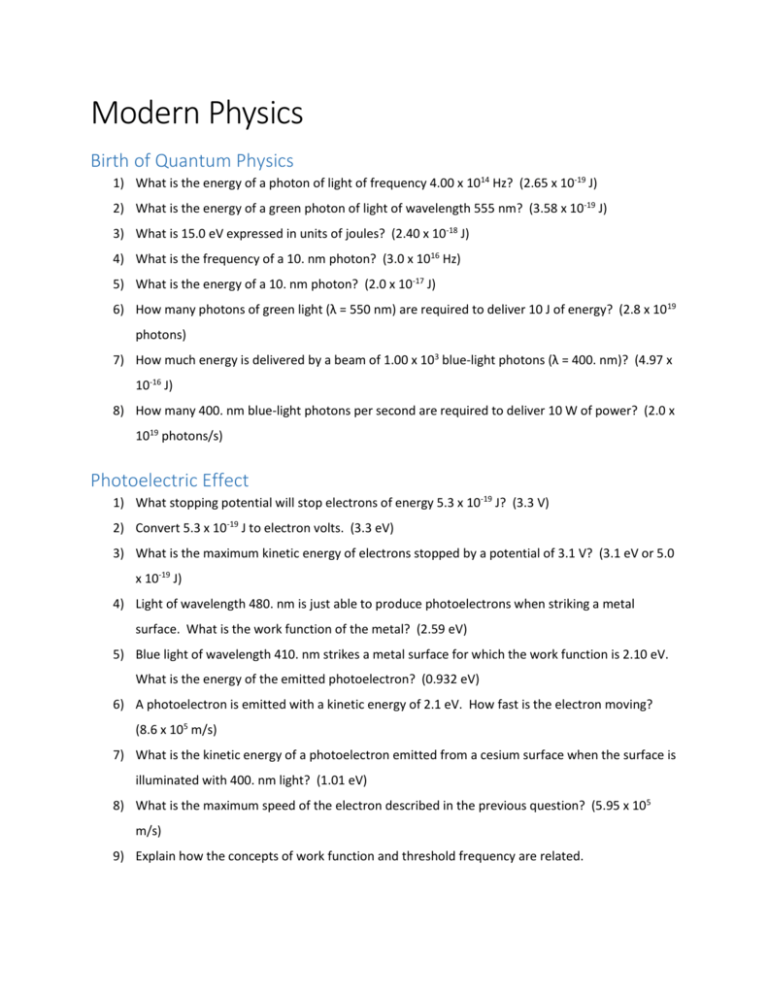
Modern Physics Birth of Quantum Physics 1) What is the energy of a photon of light of frequency 4.00 x 1014 Hz? (2.65 x 10-19 J) 2) What is the energy of a green photon of light of wavelength 555 nm? (3.58 x 10-19 J) 3) What is 15.0 eV expressed in units of joules? (2.40 x 10-18 J) 4) What is the frequency of a 10. nm photon? (3.0 x 1016 Hz) 5) What is the energy of a 10. nm photon? (2.0 x 10-17 J) 6) How many photons of green light (λ = 550 nm) are required to deliver 10 J of energy? (2.8 x 1019 photons) 7) How much energy is delivered by a beam of 1.00 x 103 blue-light photons (λ = 400. nm)? (4.97 x 10-16 J) 8) How many 400. nm blue-light photons per second are required to deliver 10 W of power? (2.0 x 1019 photons/s) Photoelectric Effect 1) What stopping potential will stop electrons of energy 5.3 x 10-19 J? (3.3 V) 2) Convert 5.3 x 10-19 J to electron volts. (3.3 eV) 3) What is the maximum kinetic energy of electrons stopped by a potential of 3.1 V? (3.1 eV or 5.0 x 10-19 J) 4) Light of wavelength 480. nm is just able to produce photoelectrons when striking a metal surface. What is the work function of the metal? (2.59 eV) 5) Blue light of wavelength 410. nm strikes a metal surface for which the work function is 2.10 eV. What is the energy of the emitted photoelectron? (0.932 eV) 6) A photoelectron is emitted with a kinetic energy of 2.1 eV. How fast is the electron moving? (8.6 x 105 m/s) 7) What is the kinetic energy of a photoelectron emitted from a cesium surface when the surface is illuminated with 400. nm light? (1.01 eV) 8) What is the maximum speed of the electron described in the previous question? (5.95 x 105 m/s) 9) Explain how the concepts of work function and threshold frequency are related.











