Complications in 1H NMR: Signal distortion in non-first
advertisement
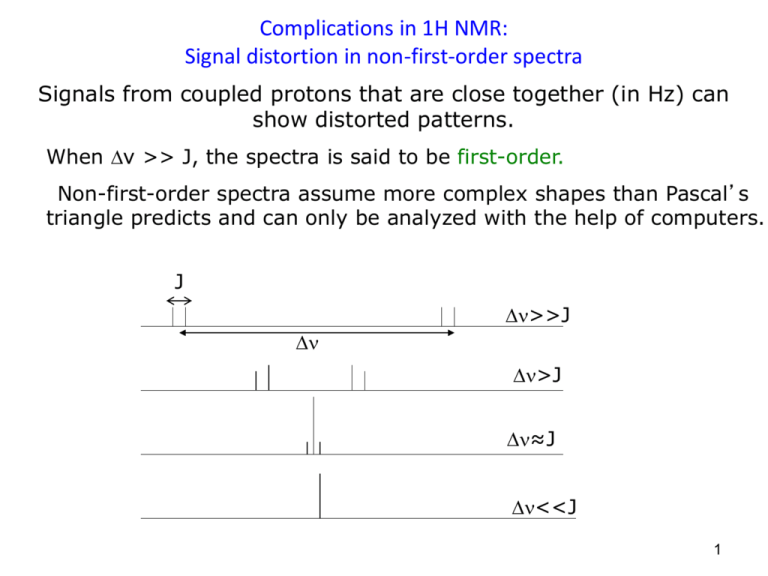
Complications in 1H NMR: Signal distortion in non-first-order spectra Signals from coupled protons that are close together (in Hz) can show distorted patterns. When ν >> J, the spectra is said to be first-order. Non-first-order spectra assume more complex shapes than Pascal’s triangle predicts and can only be analyzed with the help of computers. J >>J >J ≈J <<J 1 Top spectrum taken at 90 MHz ≈ 0.3x90 = 2.7 Hz apart Bottom spectrum taken at 500 MHz ≈ 0.3x500 = 15.0 Hz apart 2 Complications: OH groups are typically singlets (broad) Frequently protons attached to hydroxy- or aminofunctional groups do not show coupling and can appear broad. 3 For Hydroxyl protons, the lack of splitting is the result of “fast proton exchange.” Proton exchange may be slowed or stopped by removal of traces of water or acid or by cooling. This allows observation of coupling in accordance with the n+1 rule. 4 Non-first-order Spectra are common at lower Magnetic Fields 5 NON-H Nuclei: The proton nucleus possesses a greater magnetic moment and magnetic resonance frequency than do other spin-active nuclei. Below is a sketch of resonant frequencies for some common nuclei. This is not a real spectrum, since the NMR instrument is tuned to acquire a signal from just one nucleus at a time. 6 Fourier Transform NMR excites just one nucleus at a time (ie 1H nucleus @ 300,000,000 ± 3000 Hz) 7 Carbon-13 Nuclear Magnetic Resonance •13C resonate at lower E (they are less sensitive to H0) In a 7.05 T magnet where 1H’s resonate at 300 MHz, 13C’s resonate at just 75 MHz •13C NMR spectra have much lower S/N than 1H NMR spectra •13C NMR spectra typically lack the coupling information found in 1H NMR spectra •13C NMR spectra have a larger chemical shift range than 1H NMR spectra Is the range larger in Hz? Typical 13C spectra are analyzed for chemical shift and # signals only! (Integrals and Multiplicity are not reliable/ available) Range in Hz: (13C: 200 ppm = 200x75 = 1500 Hz); 1H: 12 ppm = 12x300 = 360 Hz) 8 9 Chemical Shifts in 13C NMR 13C signals are typically well resolved from one another The chemical shifts of carbon atoms in 13C NMR depend on the same effects as the chemical shifts of protons in 1H NMR. 10 Attached protons couple to 13C nuclei and complicate spectra 11 The splitting is removed through an electronic method called “broad-band decoupling”: In broad-band decoupling a pulse is applied to the proton range causing rapid - flips of hydrogen nuclei, and effectively averaging their local magnetic field contributions. Using this technique simplifies the spectra of bromoethane to two single lines. 12 Recall coupling is mutual. Why don’t we see proton signals split by their attached carbons? Carbon-carbon coupling is not visible in 1H NMR spectra due to the very low probability of two 13C nuclei being adjacent to each other in a single molecule (.0111 x .0111 ~ .0001). 13 Decoupling enhances some 13C signals more than others, and so areas no longer correspond to the number of nuclei present. 14 Why is the S/N so low for 13C NMR? •Because of the low abundance of 13C •It has a weaker inherent magnetic resonance (1/6000 as strong as 1H) •Its lower E means fewer nuclei showing net absorption. When the energy difference is small, so is the population difference between the two spin states. The smaller the E, the weaker the signal. ooooo oooooo oooooo o oooooo oooooo oooooo Weak H0 ooo oooooo oooooo ooo ooooooo ooooooo ooooooo Strong H0 15 Diastereotopic Protons 16 Diastereotopic Nuclei 17 Problems 18 Problems 19 20 21