NMR Lab
advertisement

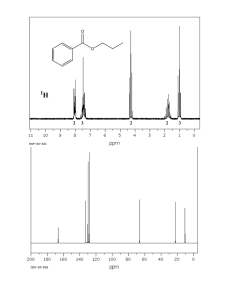
Brittany Hicks Experiment 1: NMR Lab: Becoming an Expert February 18, 2013 Introduction The Bruker 300MHz FT-NMR is a very powerful instrument that aids researchers in identifying and determining structures of organic compounds. NMR stands for nuclear magnetic resonance spectroscopy. NMR uses a magnet to detect the spins of either hydrogens or carbons in an organic molecule. NMR can only detect hydrogens or carbons because they both have an odd atomic mass. This odd atomic mass leads to a nuclear spin. The nuclei of the substance being ran, some organic compound, can further be investigated by this instrument. The NMR can be used for multiple applications, but absorbtion of electromagnetic radiation is the most common for determining different functional groups. Purpose The purpose of this lab is to learn how to become more familiarized with the instrument and its operating technique. This technique will be applied to run various samples of three known oils (peanut, vegetable, and olive), two known acids (palmitic and stearic), and two unknown samples. The unknown samples will be compared in respect to the known substance spectra obtained and analysis will be conducted. Procedure/ Methodology Preparing the sample 1. 2. 3. 4. 5. Using a small glass pipette, insert the tip into the desired sample Add about 4 drops if sample is of liquid nature or 80mg if sample is solid to the NMR tube Using a clean pipette, collect approx. 1mL of Chloroform (CDCl3) Cap the NMR sample Label what sample and solvent were used Follow SOP of NMR posted on website Data Samples were prepared for all three known oils (vegetable, olive and peanut) using chloroform as the desired solvent. Spectra ran for these samples include 13C APT, 13C, and 1H. Samples were prepared for both known acids (palmitic and stearic) using chloroform as the desired solvent. Spectra ran for stearic acid included 13C APT, 13C and 1H. For palmitic acid, we ran a 2D spectrum along with a 1H proton scan. Samples were prepared with two unknowns (A and B) using chloroform as the desired solvent. For both of these unknown samples, 13C APT, 13C, and 1H were run so that proper analysis could be observed. ALL SPECTRUM DATA ARE LABELED AND PASTED IN INSTRUMENTAL NOTEBOOK Conclusion The NMR is an instrument often used to aid in the characterization of unknown organic compounds. The spectra were difficult to interpret due to striking similarities among multiple known peaks present in a single unknown spectrum (A and B). With these comparisons however, it is likely to determine that Unknown A is potentially olive oil and Unknown B is potentially vegetable oil. The evidence that lead to these possibilities were the similarities among peaks picked. Both of the Unknown spectra were close in numbers which made it more difficult to interpret. The 2D spectrum took too long to create an appropriate scan for every sample, but after researching the purpose of a 2D spectrum, the goal was essentially to double check to make sure the carbons and hydrogens matched up. The spectra’s not completed might have aided in determining the unknowns and providing extra assurance.