Selected answers to Homework #6: NMR theory, equivalent nuclei
advertisement
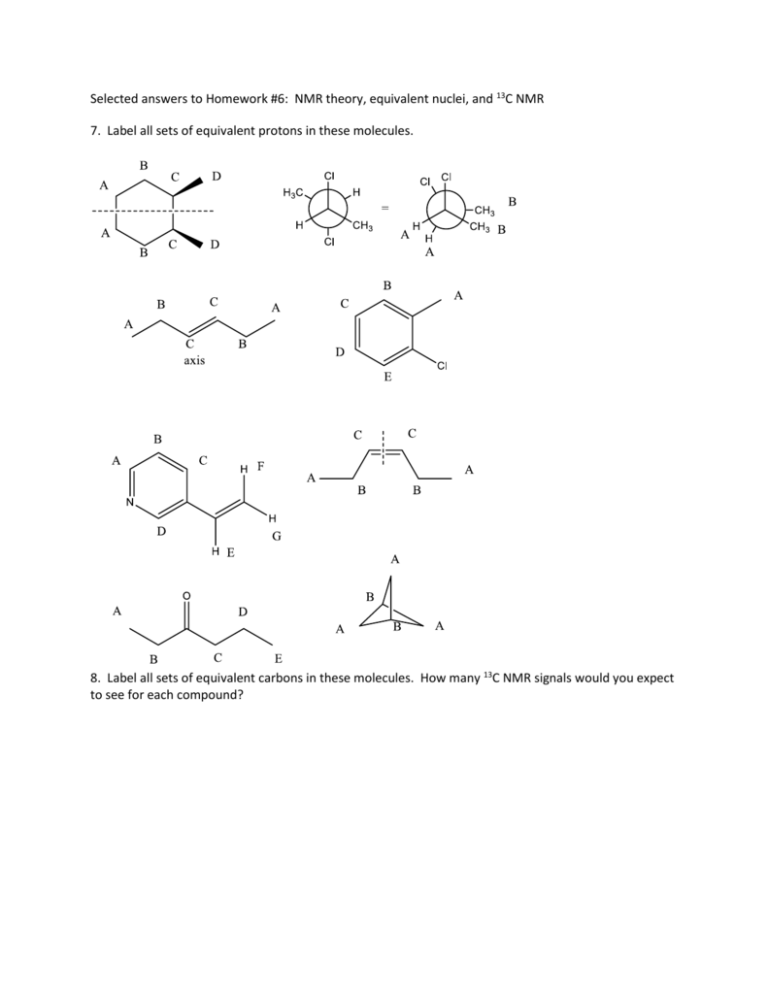
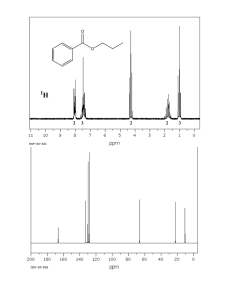
Selected answers to Homework #6: NMR theory, equivalent nuclei, and 13C NMR 7. Label all sets of equivalent protons in these molecules. 8. Label all sets of equivalent carbons in these molecules. How many 13C NMR signals would you expect to see for each compound? 9. Give estimated 13C NMR chemical shift ranges for the carbons in these molecules. Draw a predicted 13 C NMR spectrum for each compound. 10. The following elimination reactions were carried out, and the major and minor products were isolated. Would 13C NMR data alone be able to differentiate the identity of the major and minor products? 11. The following compound has 13C NMR signals at 29, 34, 134, 164, and 210 ppm. Which signals correspond to carbons A and B? Give a physical explanation for your prediction. 12. In this rearrangement, the product that is formed as one less 13C signal than the starting material. Why? Draw predicted 13C NMR for starting material and product. 13. For the following molecules, mark each set of equivalent protons, then give an approximate chemical shift for that signal. 14. Do the same as problem 1, but consider any additive effects. 17. For the compounds below, indicate sets of equivalent protons. For each set, give its integration and multiplicity. Example: 18. How many protons are represented by each signal in this spectrum of a C7H14O2 compound? Add all integrations and divide by number of Hs (12+23+25+35+37+35)/14 = 12 Divide each signal by 12 to get the number of Hs represented. 19. How many protons are represented by each signal in this spectrum of a C8H16 compound?