Stereochemistry & Chiral Molecules
advertisement
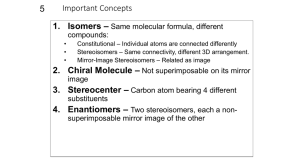
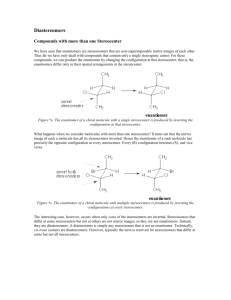
Stereochemistry & Chiral Molecules Isomerism • Isomers are different compounds with the same molecular formula • 1) Constitutional isomers: their atoms are connected in different order • 2) Stereoisomers: their atoms are connected in the same order, but have different arrangement in space – enantiomers and diastereomers (geometric isomers) Isomers Stereoisomers Enantiomers (nonsuperimposable mirror images of each other) Constitutional Isomers Diastereomers (not mirror images of each other) Stereoisomers • 1) Enantiomers: If two molecules are enantiomers of each other, one is nonsuperimposable mirror image of the other enantiomer • 2) Diastereomers: If two molecules are diastereomers, they are not mirror image of each other, but they still have the same atomic connectivity (such as cis-trans isomerism) Examples of stereoisomers diastereomer same enantiomer Enantiomers and Chiral Molecules • A chiral molecule is a molecule that is not identical with its mirror image • A chiral molecule and its mirror image are enantiomers • Enantiomers are related to each other in the same way left hand is related to right hand (in this sense, hand is chiral) • If a molecule is not chiral, it is called achiral (achiral molecule is superimposable on its mirror image) https://www.mun.ca/biology/scarr/Amino_Acid_stereoisomers.html How do we know if a molecule is chiral and have enantiomers? • One way is to look for a tetrahedral atom in the molecule that has 4 different groups attached to it • A chiral molecule does not have a plane of symmetry • Interchanging any two groups of the tetrahedral atom converts one enantiomer into the other • This carbon atom is called stereocenter/asymmetric carbon • If the tetrahedral atom has two or more of the same groups attached, it is not a stereocenter • For a molecule to be chiral, it needs to have at least one stereocenter (asymmetric carbon) Biological Significance of Chirality • Most of the molecules that make up living organisms are chiral – mostly one form of the chiral molecule is found • Amino acids (except one), building blocks of proteins, are all chiral (Lamino acids – left handed) • Natural sugars are almost all chiral (Dsugars, right handed) Nomenclature of Enantiomers • (R-S) system (also called Cahn-Ingold-Prelog system) – one enantiomer is called R, the other is called S • (R) or (S) designation is decided based on certain rules: • In biology, D, L designation is used L - (S) D – (R) Rules of (R-S) System • 1) Each of the 4 groups attached to the stereocenter is given a priority, based on the atomic number of the directly attached atom to the stereocenter • 2) If a priority can’t be assigned based on the first atom that is directly attached to the stereocenter, we look at the next set of atoms in the unassigned groups until we can make a decision • 3) After priorities are determined for all 4 groups, we rotate the molecule so that the group with lowest priority is directed away from us. For the 3 groups turned to us, we make a circle from highest priority to the lowest. If the direction of the circle is clockwise, the molecule is (R) enantiomer, if it is counterclockwise, it is (S) enantiomer. • 4) If the groups contain double or triple bonds, we assign the priorities as if both atoms were duplicated or triplicated Properties of Enantiomers: Optical Activity • Enantiomers have identical chemical and physical properties (melting point, boiling point, solubility etc.) – unlike diastereomers • There are two main differences between enantiomers: • 1) Enantiomers show different properties only when they interact with other chiral molecules (with other enantiomers) For example, the solubility of enantiomers will be different in a chiral solvent • 2) Interaction with plane-polarized light – enantiomers rotate the plane of plane-polarized light in opposite directions (that is why they are called “optically active compounds”) Interaction with Plane-polarized Light http://www.wiredchemist.com/chemistry/instructional/an-introduction-to-chemistry/structure/geometric-and-optical-isomers Biological Significance of Chirality • Chirality is important in interaction of chiral molecules with chiral receptor sites or enzyme active sites - can effect human beings differently: Limonene: one enantiomer is responsible for the odor of orange, the other enantiomer is for the odor of lemons • Activity of drugs containing stereocenters might vary between enantiomers; one enantiomer might cure, but the other might be toxic Racemic Mixture • An equimolar mixture of two enantiomers is called a racemic mixture (50% R, 50% S enantiomer) • A racemic mixture shows no rotation of planepolarized light Molecules with Multiple Stereocenters • Many of the biological organic molecules contain more than one stereocenter (cholesterol contains 8 stereocenters) • Rule for number of stereoisomers: - The total number of stereoisomers can be maximum 2n , where n is the number of tetrahedral stereocenters Diastereomers • Two stereoisomers which are not enantiomers of each other are diastereomers (have different physical properties) http://chemwiki.ucdavis.edu/Organic_Chemistry/Organic_Chemistry_With_a_Biological_Emphasis/Chapter_03%3A_Conformations_and_Stereochemistry /Section_3.7%3A_Diastereomers Meso compounds • Molecules which have a plane of symmetry are achiral, even though they contain multiple stereocenters • These compound are called meso compounds and they are optically inactive Fischer Projection Formulas • Representation of chiral molecules with twodimensional formulas • Easier to write for compounds with several stereocenters – especially used for sugars • Vertical lines represent behind or inside the plane of the paper, horizontal lines represent bonds that come out of the plane of the paper Enantiomeric Excess • If the amount of one enantiomer is greater than the other enantiomer in a mixture, such mixture is said to have enantiomeric excess • 100 % (R) – enantiomeric excess of 100 % • 70 % (R) + 30 % (S) – enantiomeric excess of 40 % • 50 % (R) + 50 % (S) – racemic mixture • Optical rotation will be in proportion to the percentage of enantiomeric excess