Organic chemistry and Biological chemistry for Health Sciences
advertisement

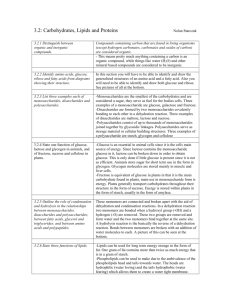
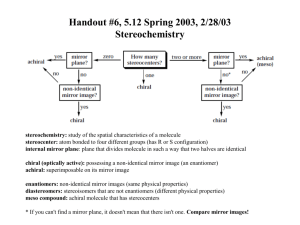
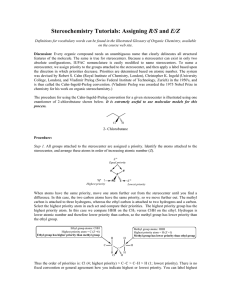
Organic chemistry and Biological chemistry for Health Sciences 59-191 Lecture 14 Synthesis of amides: Amides can be made from amines by acyl group transfer reaction from acid derivatives. Either acid chloride or acid anhydride reacts smoothly with amonia or amines to give amides. In the body, other kinds of acyl group carrier molecules serve instead of ordinary acid chlorides and anhydrides as sources of the acyl group. When proteins are made from amino acids, for example, the acyl portion of amino acids-they are called aminoacyl units-are held by carrier molecules. When a cell makes an amide bond, it transfers an aminoacyl group from its carrier molecule to the nitrogen atom of the amino group. The carrier molecule is released to be reused. CARBOHYDRATES: Monosaccharides: Monosaccharides or simple sugars are carbohydrates that cannot be hydrolyzed. The names of monosaccharides end in –ose. The monosaccharides, which contain aldehyde group, are called aldose and those containing ketone groups are called ketose. Monosaccharides (aldose or ketose) with three carbons are called triose, those with four carbons tetraose and so on. Two trioses, glyceraldehyde and dihydroxyacetone, occur in metalbolism. Two pentoses, ribose and deoxyribose, are needed by nucleic acids. The nutritionally important monosaccharides are all hexoses. Glucose is a hexose with an aldehyde group, called aldohexose. Galactose is a stereoisomer of glucose is also an aldohexose. Fructose is a ketohexose and a constitutional isomer of glucose and galactose. Disaccharides are carbohydrates that can be hydrolyzed to two monosaccharides. F.example sucrose (table sugar), maltose, and lactose . Polysaccharides can be hydrolyzed in water to give hundreds of monosaccharides. F.exp. starch, cellulose. Carbohydrates that give positive Tollen’s test and positive Benedict’s test are called reducing sugars. All monosaccharides and nearly all disaccharides are reducing sugars. But sucrose is not a reducing sugar and neither the polysaccharides. All aldohexoses, including glucose, have 2,3,4,5,6-pentahydroxyhexanal structure. Carbons 2,3,4, and 5 in the glucose chain are all tetrahedral stereocenters. Each center has unique set of four different groups. Those carbons are also called chiral carbon. Compounds having chiral carbon are optically active. They can rotate plane polarized light. The degree of rotation of the plane of plane-polarized light caused by an optically active solution is called optical rotation. It is usually expressed by using the symbol . The number of stereoisomers of a compound whose molecules have n differnent tetrahedral stereocenters is 2n. In 2,3,4,5,6-pentahydroxyhexanal, n equals four, so there must be 24 or 16 stereoisomers, occuring as eight pairs of enantiomers. Glucose is one of those 16; galactose is another. Enantiomers are stereoisomers whose molecules are related as an object is related to its mirror image but that cannot be superimposed. Stereoisomers whose molecules are not related this way are called diestereomers. Absolute configurations of the enantiomers of glyceraldehydes are used to devise configurational or optical families for the rest of the carbohydrates. Any compound that has a configuration like that of (+) glyceraldehyde (D-glyceraldehyde) is said to be in Dfamily. When the molecules of a compound are the mirror images of an enantiomer in the Dfamily, the compound is in the L-family. The letters D and L are only family names. They have nothing to do with actual signs of the values of their specific optical rotation. When a molecule has many stereocenters, it becomes quite complicated to make a perspective, three dimensional drawing of an absolute configuration. Emil Fisher, a chemist devised an easy way to project three-dimensional configuration of each stereocenter in a molecule onto a plane surface. His structural representations are called Fisher projection formulas. The following rules are used to write the fisher projection formulas: Visualize the molecule with its main carbon chain vertical and with the bonds that hold the chain together projecting to the rear at each stereocenter. Carbon 1 is at the top. Mentallly flatten the structure, stereocenter by stereocenter, onto a plain surface. In the projected structure, represent each stereocenter either as the intersection of two lines or conventionally as C. The horizontal lines at a stereocenter actually represent bonds that project forward, out of the plane of the paper and the vertical lines represent bonds that project rearward, behind the plane A fisher projection formula can have more than one intersection of lines, each representing a stereocenter. At each stereocenter, a horizontal line is a bond coming toward you and a vertical line is a bond going away from you.