MOLSREVIEWJune_3
advertisement
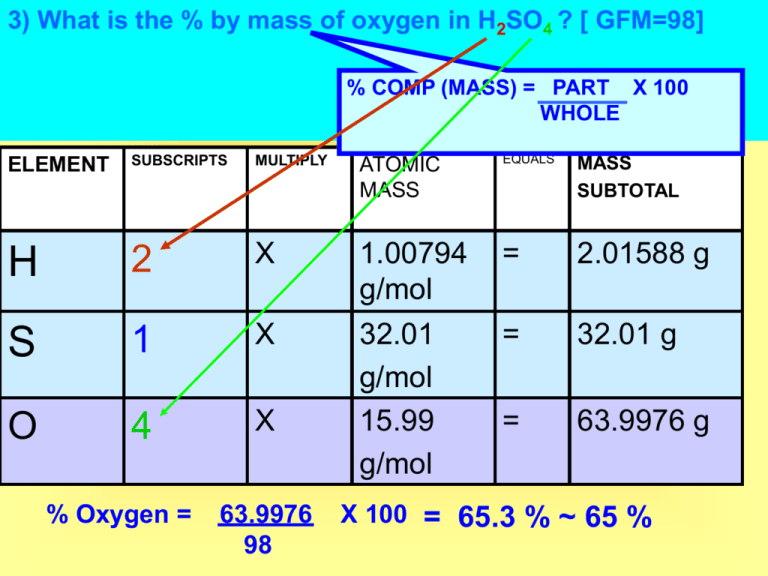
3) What is the % by mass of oxygen in H2SO4 ? [ GFM=98] % COMP (MASS) = PART X 100 WHOLE ELEMENT SUBSCRIPTS MULTIPLY ATOMIC MASS EQUALS MASS SUBTOTAL H 2 X = 2.01588 g S 1 X = 32.01 g O 4 X 1.00794 g/mol 32.01 g/mol 15.99 g/mol = 63.9976 g % Oxygen = 63.9976 98 X 100 = 65.3 % ~ 65 % #5) A hydrate is a compound that includes water molecules within its crystal structure. During an experiment to determine the percent by mass of water in a hydrated crystal, a student found the mass of the hydrated crystal to be 4.10 grams. After heating to constant mass, the mass was 3.70 grams. What is the percent by mass of water in this crystal? Mass water % H2O = Mass hydrate x 100 = 4.10 g – 3.70 g % H2O = This is a modified version of part/whole x 100 from the reference tables! 4.10 g x 100 = 9.8 % water 7) Given the reaction: 6 CO2 + 6 H2O 1 C6H12O6 + 6 O2 a) What is the total number of moles of water needed to make 2.5 moles of C6H12O6? H2O . . C6H12O6 . Theoretical mole ratio 6 1 = x 2.5 X = 15 moles of water #13) A compound contains 0.5 moles of sodium, 0.5 moles of nitrogen, and 1.0 moles of hydrogen. The empirical formula of the compound is Na0.5N0.5H1.0 Multiply subscripts by 2 Subscripts can not be decimals, to get rid of a 0.5 decimal multiply all subscripts by 2! Na0.5x2N0.5x2H1.0x2 The new subscripts are: Na1N1H2 or NaNH2 #15) A compound contains 40% CALCIUM, 12% CARBON and 48% OXYGEN by mass. What is the empirical formula of this compound? ELEMEN T MASS / ATOMIC MASS RAW RATIO Ca 40 g / 40.08g/mol =0.998 / 0.998 = 1.0 C 12 g / 12.01g/mol =0.999 / 0.998 = 1.0 O 48 g / 15.99g/mol =3.00 / 0.998 = 3.0 Assume 100g of the sample, this will allow you to assume 40% is 40 grams. Total mass does NOT affect % composition. DIVIDE BY SMALLEST Ca1C1O3 CaCO3 SUBSCRIPT RATIO