Nick Tang 2nd Period
advertisement

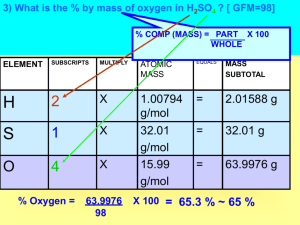
Chapter Three: Stoichiometry Nick Tang 2nd Period Ms. Ricks 3.1 Chemical Equations • + means reacts with, means produces • Chemicals to the left represent reactants, Chemicals to the right represent products • The numbers in front of the formulas are coefficients while the small number on the bottom are called subscripts • The coefficients lets you know how much there is of that substance, while the subscripts indicate its identity Balancing Equations • An equation is balanced once each side of the equation has the same amount of atoms of each element • You can change the coefficients but never the subscripts • It’s usually best to try to first balance the elements that occurs the least in the equation CH4 + O2 CO2 + H2O • We have 1 C on both sides, 4 H’s on the left while 2 H’s on the right, and 2 O’s on left and 3 O’s on the right • Balance the H first on the left by putting a 2 in front of H2O and then balance the O’s by putting a 2 in front of the O2 Final equation: CH4 + 2O2 CO2 + 2H2O Check Up • Balance the following __Fe + __O2 __Fe2O3 ___C2H4 + __O2 __CO2 + __ H2O ___Al + __HCL __ALCL3 + __H2 • • • 4,3,2 1,3,2,2 2,6,2,3 Indicating the States of Reactants and Products • The 4 states are solid (s), liquid (l), gas (g), and aqueous (aq) • We use this to indicate the physical state of each substance CH4(g) + 2O2(g) CO2(g) + 2H2O(l) 3.2 Some Simple Patterns of Chemical Reactivity • Combination reactions: two or more substances react to form one product. Also when a metal combines with a nonmetal the product is an ionic solid. Ex: 2Mg + O2 2MgO • Decomposition reactions: one substance undergoes a reaction to produce two or more other substances. Ex: CaCO3 CaO +CO2 3.2 Some Simple Patterns of Chemical Reactivity Cont. • Combustion in Air. Combustion reactions are rapid reactions that produce a flame. • Most of these reactions involve O2 as a reactant • When hydrocarbons are combusted they react with O2 to make CO2 and H2O C3H8 + 5O2 3CO2 + 4H2O Check Up • Write equation for: • Solid mercury(II) sulfide decomposes into its component elements when heated • Write the balanced equation for the reaction that occurs when ethanol, C2H5OH is burned in air • HgS Hg + S • C2H5OH + 3O2 2CO2 + 3H2O 3.3 Formula Weights • The formula weight of a substance is the sum of the atomic weights of each atom in its chemical formula • Use the atomic masses from the periodic table and multiply with the subscripts and coefficients to find the weight • Ex: FW of H2SO4 = 2(AW of H) + (AW of S) + 4(AW of O) = 98.1amu • Percent Composition: How much of a element contributes to the mass of the entire substance. Use this equation Check Up • Calculate the formula weight of sucrose C12H22O11 • Calculate the percentage of nitrogen, by mass, in Ca(NO3)2 • 342.0 amu • 17.1% 3.4 Avogadro’s Number and the Mole • Mole(abbreviated mol) is the unit for dealing with atoms, ions, or molecules • Avogadro’s number is 6.02E23 • Molar Mass the mass in grams of one mole of a substance • Make sure you know how to convert moles to atoms, grams to moles, moles to grams, and number of molecules and number of atoms from mass Converting Moles to Number of Atoms Calculate the number of H atoms in 0.350 mol of C6H12O6 First put how many moles you have and convert it to molecules. We know we have 6.02E23 molecules for every mole so multiply by 6.02E23 and then multiply by how many H atoms there are which in this case is 12 There are 2.53E24 H atoms Converting grams to moles Calculate the number of moles of glucose (C6H12O6) in 5.380 g of C6H12O6 First put how much you have of the substance and then divide by it’s molar mass and multiply by 1 mol Put that in the calculator and you should get .02989 mol C6H12O6 Converting moles to grams Calculate the mass, in grams, of 0.433 mol of calcium nitrate First put down how many moles of calcium nitrate you have and we know that one mol is equivalent to the molar mass of the compound so multiply .433mols of Ca(NO3)2 by its molar mass, 164.1g Ca(NO3)2 Answer: 71.1g Ca(NO3)2 Check Up Super Problem: How many glucose molecules are in 5.23g of C6H12O6? How many oxygen atoms are in this sample? • 1.75E22 molecules C6H12O6 • 1.05E23 atoms O 3.5 Empirical Formulas from Analyses • The empirical formula tells us the simplest whole number ratio of moles of each element in a compound • To find the empirical formula, first take the given masses of each element and divide by their atomic masses • Afterwards determine the simplest whole number ratio of moles by dividing each number of moles by the smallest number of moles • If you get decimals either round them if they are close(.9, .1) but if it’s too far from being a whole number such as 1.5 multiple it(and everything else) by a ratio to make it even 3.5 Empirical Formulas from Analyses Cont. What is the empirical formula of vitamin C which contains 40.92% C, 4.58% H, and 54.50% O by mass 3.5 Empirical Formulas from Analyses Cont. • Molecular formula indicates the actual number of atoms of each element in one molecule of a substance • To find the molecular formula you must have the molecular weight and the empirical formula weight • You divide the molecular weight by the empirical formula weight to find your whole number multiple, which you use to multiply the subscripts in the empirical formula molecular weight Whole-number multiple= -------------------------------empirical formula weight 3.5 Empirical Formulas from Analyses Cont. Determine the molecular formula of C3H4 which has a molecular weight of 121 amu 3.5 Empirical Formulas from Analyses Cont. • Combustion analysis is commonly used for compounds containing carbon and hydrogen as their component elements • CO2 and H2O are produced and from the masses of CO2 and H2O we can calculate the empirical formula • If there is a third element we can find it’s mass by subtracting the total mass by the mass of C and H Combustion Analysis Combustion of 0.255g of isopropyl alcohol produces 0.561g of CO2 and 0.306g of H2O. Determine the empirical formula Combustion Analysis Cont. 3.6 Quantitative Information from Balanced Equations 2H2 + O2 2H2O • The quantities 2 mol H2, 1 mol O2, and 2 mol H2O are called stoichiometric ally equivalent quantities • This shows the stoichiometric relations can can be used to convert between quantities of reactants and products in a chemical reaction 3.6 Quantitative Information from Balanced Equations How many grams of water are produced in the oxidation of 1.00g of glucose, C6H12O6? C6H12O6 + 6O2 6CO2 + 6H2O 3.7 Limiting Reactants • The limiting reactant is the reactant that is completely consumed and determines the amount of product formed • The other reactant is called the excess reactant which is what is left over N2 + 3H2 2NH3 How many moles of NH3 can be formed from 3.0 mol of N2 and 6.0 mol of H2 3.7 Limiting Reactants Conti. • We don’t have 9 moles of H2 therefore H2 is the limiting reactant • Now go from moles of H2 to moles of NH3 3.7 Theoretical Yields • Theoretical yield is the quantity of product that is calculated to form when all of the limiting reactant reacts • The amount of product actually obtained in a reaction is the actual yield • The actual yield is almost always less than the theoretical due to some parts of the reactants not reacting or some other outside force but never will it be higher than the theoretical • Percent yield relates the actual to the theoretical using this equation Actual Yield Percent Yield= ---------------------------- X 100% Theoretical Yield 3.7 Theoretical Yields Cont. 2C6H12 + 5O2 2H2C6H8O4 + 2H2O Assume that you carry out this reaction starting with 25g of C6H12 and that C6H12 is your limiting reactant. What’s the theoretical yield of 2H2C6H8O4? If you obtain 33.5g of 2H2C6H8O4 what is the percent yield for 2H2C6H8O4?