Using Chemical Formulas - Belle Vernon Area School District

7.3
Formula Mass – for any molecule, formula unit, or ion, it is the ____ of the ________ masses of all atoms represented in its formula.
H
2
O for example is
H = 1.008 x 2 = 2.016 amu
O = 15.999 x 1 = 15.999 amu
Total = 18.015 amu
Try to find the mass of
KClO
3
Ca(NO
3
)
2
MgCl
2
H
2
SO
4
PO
4
3-
The mass of 1 mol of a substance in g/mol
H
2
O for example is
H = 1.008 x 2 = 2.016 g/mol
O = 15.999 x 1 = 15.999 g/mol
Total = 18.015 g/mol
The ___ mass is numerically = to the ___ mass
You can convert grams to moles and vice versa using ______ ______
You can also convert atoms to moles and vice versa using ___________ #
What is the mass in grams of 2.50 mol of oxygen gas?
How many moles are in 100 g of H
2
O?
How many moles are in 10 grams of
Carbon?
How many moles are in 3.01 x 10 23 molecules of CH
4
?
How many atoms are in 15 moles of S?
How many molecules are there in the following…
25.0 g H
2
SO
4
125 g Sugar C
6
H
12
O
6
How many grams are in…
6.5 x 10 23 atoms of Cu
5.12 x 10 30 molecules of CH
4
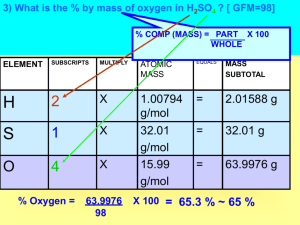
Percent Composition – the % by mass of each _________ in a cmpd
To calculate % Comp, determine
_______ mass of entire compound.
Calculate the % of each element by
__________ the mass of each
___________ by the molar mass.
Determine the percent composition of Al and O in
Al
2
O
3
.
Determine the composition of
Al, S, and O in Al
2
(SO
4
)
3
Find the % comp of each element in the following
PbCl
2
Ba(NO
3
)
2
Find the % water in ZnSO4•7H
2
O
Mg(OH)
2 is 54.87% O by mass. How many g of O are in 175 g of the cmpd?
How many moles of O is this?
Complete the section review on page 244 numbers 1-6. This is due at the beginning of class tomorrow!