Mole Review Problems (Be sure to SHOW ALL WORK and circle
advertisement

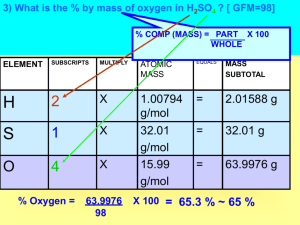
Mole Review Problems (Be sure to SHOW ALL WORK and circle your final answer for each problem) The mass of 1 mol of lead atoms is 207.2 g. Find the mass of 1 atom of Pb 3.44 x 10-22 g/atom You have 1.6 x 1021 molecules of nitrogen gas, N2. How many moles? 2.66 x 10-3 mol What is the mass of 2.5 moles of silicon? 70.21 g How many moles of aluminum are present in 180.0 g Al? 6.67 mol How many moles of chlorine atoms are present in 2.0 grams of chlorine gas, Cl2? 0.0564 mol Cl atoms A compound with a mass of 48.72 g is found to contain 32.69 g of zinc and 16.03 g of sulfur. What is the percentage composition of the compound? 67% Zn, 33% S Calculate the percent composition of H2O 11.2% H, 88.8% O Calculate the percent composition of K3PO4 K = 55.258%, P = 14.592%, O = 30.150% Calculate the percent composition of TeCl2 Te = 64.280%, Cl = 35.720% A sample of a compound is analyzed and found to contain 0.90 g of calcium and 1.60 g of chlorine. The sample has a mass of 2.50 g. Determine the percentage composition of the compound Ca = 36%, Cl = 64% Mole Review Problems (Be sure to SHOW ALL WORK and circle your final answer for each problem) The mass of 1 mol of iron atoms is 55.85 g. Find the mass of 1 atom of Fe 9.277 x 1023 g/atom You have 1.6 x 1021 molecules of fluorine gas, F2. How many moles? 2.66 x 10-3 mol What is the mass of 2.5 moles of gallium? 174g How many moles of potassium are present in 180.0 g K? 4.60 mol How many moles of silver atoms are present in 25.0 grams of Ag? 0.232 mol A compound is composed of 36.475% sulfur and 63.525% iron. What is the empirical formula? FeS Calculate the percent composition of Fe(NO3)2 Fe = 31.05%, N = 15.576%, O = 53.374% Calculate the percent composition of Na3PO4 Na = 42.070%, P = 18.893%, O = 39.037% Calculate the percent composition of OsCl2 Os = 72.847%, Cl = 27.153% A sample of a compound is composed of 20.090% sulfur, 39.814% copper, and 40.097% oxygen. What is the empirical formula? CuSO4