AP Chemistry Semester Exam
advertisement

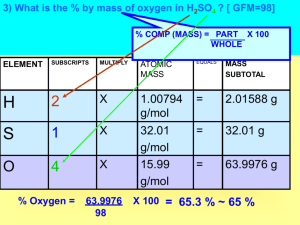
AP Chemistry First Semester Exam Multiple Choice. You may use a calculator with this section. ______1. How many grams of calcium nitrate, Ca(NO 3)2, contains 24 grams of oxygen atoms? a. 164 grams b. 96 grams c. 62 grams d. 50. grams e. 41 grams _______2. The simplest formula for an oxide of nitrogen that is 36.8 percent nitrogen by weight is: a. N2O b. NO c. NO2 d. N2O3 e. N2O5 ______3. A 2.00 liter sample of nitrogen gas at 27 oC and 600. millimeters of mercury is heated until it occupies a volume of 5.00 liters. If the pressure remains unchanged, the final temperature of the gas is: a. 68 oC b. 120 oC c. 477 oC d. 677 oC e. 950. oC ______4. When a hydrate of Na2CO3 is heated until all the water is removed, it loses 54.3 percent of its mass. The formula of the hydrate is: a. Na2CO3 10 H2O b. Na2CO3 7 H2O c. Na2CO3 5 H2O d. Na2CO3 3 H2O e. Na2CO3 H2O 5. When the equation for the half-reaction below is balanced, what is the ratio of the coefficients OH-/CrO2-? CrO2- + OH- CrO4-2 + H2O + ea. 1:1 b. 2:1 c. 3:1 d. 4:1 e. 5:1 6. Which of the following statements regarding the reaction represented by the equation below is correct? 6 I- + 2 MnO4- + 4 H2O (l) 3 I2 (s) + 2 MnO2 (s) + OH- 8. a. Iodide ion is oxidized by the hydroxide ion. b. MnO4- is oxidized by the iodide ion. c. The oxidation number of manganese changes from +7 to +2. d. The oxidation number of manganese remains the same. e. The oxidation number of iodine changes from –1 to 0. When the equation for the half reaction below is balanced with the lowest whole-number coefficients, the coefficient for H2O is : Cr2O7-2 + a. b. c. d. 2 4 6 7 e- + H+ Cr+3 + H2O (l) e. 14 9. What is the molarity of 125.0 g of NaOH in 500.0 ml of solution? a. 3.125 M b. 2.00 M c. 6.250 M d. 1.250 M e. 6.0 M 10. The net ionic equation for the reaction that occurs during the titration of nitric acid with sodium hydroxide is: a. HNO3 + Na+ + OH- NaNO3 + H2O b. HNO3 + NaOH Na+ + NO3- + H2O c. H+ + OH- H2O d. HNO3 + H2O NO3- + H3O+ e. HNO3 + OH- NO3- + H2O 11. A yellow precipitate forms when 0.5 M NaI (aq) is added to a 0.5 M solution of which of the following ions? a. Pb+2 (aq) b. Zn+2 (aq) c. CrO4-2 (aq) d. SO4-2 (aq) e. OH- (aq) ______ 12. Which of the following represents a pair of isotopes? (1984, 19) Atomic Mass Number Number A I. 6 14 II. 7 14 B I. 6 7 II. 14 14 C I. 6 14 II. 14 28 D I. 7 13 II. 7 14 E I. 8 16 II. 16 20 _______13. Which of the following represents the ground state electron configuration of the Mn+3 ion? (Atomic number Mn = 25) a. 1s22s22p63s23p63d4 b. 1s22s22p63s23p63d54s2 c. 1s22s22p63s23p63d24s2 d. 1s22s22p63s23p63d84s2 e. 1s22s22p63s23p63d34s24p2 14. A correct ranking of elements in order of increasing electronegativity is: a. Ca-Li-Ba-K-Ca b. H-I-Na-K-Ca c. Ca-Al-P-S-F d. F-Br-H-Al-Rb e. Na-O-C-Ca-Li _______15. A compound is made of only sulfur and oxygen. It is 64.1% S by mass. Its molar mass is 192 g/mol. What is its formula? a. SO2 b. SO4 c. SO3 d. S4O4 e. S2O3 _______16. What is the density of ammonia at 25 oC and 750 torr? a. 1.3 g/liter b. 0.69g/liter c. 0.25g/liter d. 0.3 g/liter e. 6.9 g/liter Use these answers for questions 17-19. a. F b. Cr c. Na d. Mg e. Br 16. What is the most electronegative element of the elements above? 17. Which element exhibits the greatest number of different oxidation state? 18. Which of the elements above has the smallest ionic radius for its most commonly found ion? _______19. Which set of quantum numbers (n,l.ml, ms) is not possible? a. 1,0,0, ½ b. 1,1,0, ½ c. 1,0,0,-1/2 d. 2,1,-1, ½ e. 3,2,1,1/2 Use the following to answer questions 20-22. a. b. c. d. e. 1s2 2s2 2p6 3s2 3p4 1s2 2s2 2p6 3s2 3p6 4s1 3d5 1s2 2s2 2p8 3s2 3p6 1s2 2s2 2p6 3s2 3p6 4s2 3d4 1s2 2s2 2p6 3s2 3p6 _______20. What is the electron configuration of Cr? _______21. violates the Pauli Exclusion principle _______22. noble gas configuration _______23. Who sings Another Brick in the Wall? a. Pink Floyd b. Pink Floyd’s teacher c. Joe while he takes a shower. d. Mahtab would but he doesn’t sing. _______24.The number of electrons, protons, and neutrons, respectively, in the 31P isotope are: a. 15, 31, 14 b. 15, 15, 31 c. 31, 15, 16 d. 15, 15, 16 e. 31, 31, 16 _______25. The gold foil experiment was used to determine the: f. mass of an electron g. the existence of a positive nucleus h. the charge of an electron i. the presences of neutrons in the nucleus _______26. Who made the important discoveries about the properties of cathode ray tubes? j. Dalton k. Millikan l. Rutherford m. Thomson n. Chadwick ________27. Which of the following is incorrectly named? o. H2SO4, sulfuric acid p. H2CO3, carbonic acid q. HCN, cyanic acid r. H3PO4, phosphoric acid ________28. Which of the following is incorrectly paired? s. Na, alkali metal t. Mg, alkaline earth metal u. Br, halogen v. Sn, transition metal w. Ar, noble gas ________29. Which metals form cations with variable positive charges? a. Group 1 a. Group 2 b. Group 3 c. Metalloids d. Transition metals ________30. Which pair of samples contains the same number of oxygen atoms in each compound? e. 0.10 mol Al2O3 and 0.50 mol BaO f. 0.20 mol Cl2O and 0. 10 mol HClO g. 0.10 mol Na2O and 0.10 mol Na2SO4 h. 0.20 mol SnO and 0.20 mol SnO2 i. 0.2 mol Ca(OH)2 and 0.10 mol H2C2O4 ________31. Who stresses more over tests? j. Alyssa k. Alexus l. Victoria m. Hamilton _________32. When the equation for the half-reaction below is balanced, what is the ratio of the coefficients OH-/CrO2-? CrO2- + OH- CrO4-2 + H2O + ea. b. c. d. e. 1:1 2:1 3:1 4:1 5:1 ScCl3 (aq) + 3 KOH (aq) → Sc(OH)3 s + 3 KCl _________33. Which of the following identifies the maximum number of moles of products formed when 0.60 moles of SnCl3 (aq) is mixed with 0.60 moles of KOH in water? a. b. c. d. 0.20 moles of Sc(OH)3 and 0.20 moles of KCl 0.20 moles of Sc(OH)3 and 0.60 moles of KCl 0.20 moles of Sc(OH)3 and 1.20 moles of KCl 0.60 moles of Sc(OH)3 and 0.60 moles of KCl 34. A sealed container contains 0.30 moles of oxygen gas and 0.20 moles of hydrogen gas. If the temperature is 55 oC throughout the container, which of the following is true? a. b. c. d. e. The partial pressures of the two gases are the same. The average kinetic energy of the two gases are the same. The molecular masses of the two gases are the same. The total masses of the two gases are the same. The average molecular speeds of the two gases are the same. 35. A gas sample contains 0.20 moles of oxygen and 0.60 moles of nitrogen. If the sample is at standard temperature and pressure, what is the partial pressure due to nitrogen? a. 0.15 atm b. 0.25 atm c. 0.75 atm d. 0.80 atm e. 1.0 atm 36. Nitrogen gas was collected over water at 25 oC. If the vapor pressure of water at 25 oC is 23 mm Hg and the total pressure in the container is measured at 781 mm Hg, what is the partial pressure of the nitrogen gas? a. b. c. d. e. 23 mm Hg 46 mm Hg 551 mm Hg 735 mm Hg 758 mm Hg 37. A gas sample is confined in a 5 Liter container. Which of the following occur if the temperature of the container is increased? I. The kinetic energy of the gas will increase. II. The pressure of the gas will increase. III. The density of the gas will increase. a. I only b. II only c. I and II only d. I and III only e. I, II, and III Matching: Match the gas law with its definition or description. _______38. 1mole of any gas at STP occupies a volume of 22.4 L _______39. volume and pressure are inversely proportional when temperature is held constant. _______40. volume and temperature are directly proportional when pressure is held constant. _______41. The total pressure of a mixture of gases is the sum of the partial pressures. _______42. An equation of state that includes a combined proportionality constant A. Ideal Gas Law B. Charles’ Law C. Avogadro’s Law D. Dalton’s Law E. Boyle’s Law Discussion: 43. Represented above are five identical balloons, each filled to the same volume at 25C and 1.0 atmosphere pressure with the pure gases indicated. (a) Which balloon contains the greatest mass of gas? Explain. (2 points) (b) Compare the average kinetic energies of the gas molecules in the balloons. Explain. (2 points) (c) Which balloon contains molecules that would travel at the lowest molecular speed? Explain. (2 points) (d) Twelve hours after being filled, all the balloons have decreased in size. Predict which balloon will be the smallest. Explain your reasoning. (2 points) 44. (a) Write the ground state electron configuration for an arsenic atom, showing the number of electrons in each subshell. ( 2 points) (b) Give one permissible set of four quantum numbers for each of the outermost electrons in a single As atom when it is in its ground state. (2 points) (c) Is an isolated arsenic atom in the ground state paramagnetic or diamagnetic? Explain briefly. (2 points) (d) Explain how the electron configuration of the arsenic atom in the ground state is consistent with the existence of the following known compounds: Na3As, AsCl3, and AsF5. (2 points) 45. 2KClO3 2KCl + 3O2 a. What is the mass of O2 produced when 15 grams of KClO3 is heated, according to the equation above, in an open vessel until no further weight loss is observed? 46. Three students weighed the same sample of copper shot five times. Their results were as follows: weighing 1 2 3 4 5 Student #1 17.516 g 17.888 g 19.107 g 21.456 g 19.983 g Student #2 15.414 g 16.413 g 14.408 g 15.637 g 15.210 g Student #3 13.893 g 13.726 g 13.994 g 13.810 g 13.476 g a. calculate the mean (average) of each sample . b. If the true mass of the copper shot is 15.384 g, which of the students was most accurate? Which was most precise? c. calculate the % error of student # 1 47. Chlorophyll a, a photosynthetic pigment found in plants, absorbs light with a wavelength of 660 nm. a. determine its frequency b. calculate the energy of a photon of light with a wavelength of 660 nm. c. If an electron moves from n=4 to n=2 is energy absorbed or emitted? d. What is the wavelength of this spectral line. 48. AP chemistry is hard but it will: a. lead to insanity or at least a mini-breakdown b. help me in college c. make me appreciate baby chemistry d. all of the above, but Mrs. Stephens would prefer that I put b as the correct answer.