I. Introduction to Bonding

Chemical Bonding
Chapter 7
Section 1
Pages 219-231
III IV
Chemical Formulas
Chemical formulas indicate the elements involved and how many of each type are in the molecule.
C
8
H
18 subscripts
Al
2
(SO
4
)
3
Types of Ions
Monatomic Ions – one element
Criss-Cross Method
Used to write formulas of ionic compounds (salts)
Step 1: List Charges of each ion
Step 2: Criss-Cross the # of the Charge only!
Step 3: Simplify (Never write a subscript of 1)
Criss-Cross means:
•
# of the Charge of cation
•
# of the Charge of anion subscript of the anion .
subscript of the cation.
Types of Ions
Polyatomic – more than one element
Example: Calcium Phosphate
Use parentheses for a polyatomic ion.
Ca = 2+ PO
4
= 3-
Ca
3
(PO
4
)
2
Empirical Formula
Lowest Whole Number Ratio of Elements
CaSO
4
= 1 calcium ion for every 1 sulfate ion
Na
2
SO
4
= 2 sodium ions for every 1 sulfate ion
*Stock System of Naming
Compounds
Roman numeral matching the charge is used.
Ex: Cu +1 , copper I
Cu +2 , copper II
Cu +2 N0
3
: Cu(N0
3
)
2
Cu + NO
3
: CuNO
3 copper II nitrate copper I nitrate
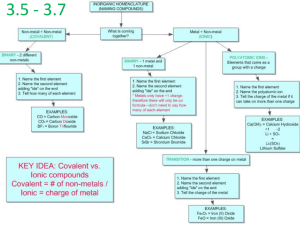
Naming Binary Ionic Compounds
Cation: Name the Metal (use Roman numerals for transition metals)
Anion: nonmetal Change the ending to –ide.
Calcium Chloride
Barium Oxide
Potassium Sulfide
Iron (III) Bromide
Chromium (III) Oxide
Traditional Naming of Ionic
Compounds
Traditional naming of ionic compounds used the
Latin names or some common names and a suffix corresponding to the charge as follows.
Copper: Cuprum
Cu +1 : cuprous Cu +2 : cupric
The ous ending was used with the lower charged ion.
The ic ending was used with the higher charged ion.
Tradition Names
Ferrous Nitrate : Iron +2, nitrate: Fe(NO
3
)
2
Plumbic Chloride: Lead +4, chlorine: PbCl
4
Latin Names:
Ferrum – Iron, Cuprum – Copper,
Stannum – Tin, Mercurum – Mercury
Common Names: Any element with more than one possible charge: lead, mercury, tin, etc.
*Examples of Naming for Polyatomic Ions
Calcium Sulfite
Barium Phosphate
Ammonium Sulfide
Iron (III) Sulfite
Naming Molecular Compounds
Same rules
EXCEPT include prefixes
NEVER start a compound name with mono-
(drop the prefix)
1 = mono 2 = di
3 = tri 4 = tetra
5 = penta 6 = hexa
7 = hepta 8 = octa
9 = nona 10 = deca
Naming Molecular Compounds
NO
2
= nitrogen dioxide
BF
3
= boron trifluoride
P
2
O
5
= diphosphorus pentoxide
*Molecular Formula
Indicates how many atoms are in a single molecule of the compound.
C
6
H
12
O
6
= glucose
6 carbon atoms
12 hydrogen atoms
6 oxygen atoms
Naming of Acids & Salts
Binary Acids: Contain only two elements.
Hydrogen & a nonmetal
Hydro- ____________ -ic acid.
Ex: HCl = Hydrochloric Acid
H
2
S = Hydrosulfuric Acid
Naming of Acids & Salts
Binary Salts: Contain only two elements.
A metal & a nonmetal
Name the metal & change the ending on the non-metal to –ide.
Ex: NaCl = Sodium Chloride
MgBr
2
= Magnesium Bromide
Naming of Acids & Salts
Ternary Acids: Prefix & Suffix
X could be any element other than (Sulfur,
Phosphorus, Nitrogen or Carbon)
HXO = hypo-________-ous
HXO
2
HXO
3
HXO
4
= __________-ous
= __________-ic
= per-_________-ic
Naming of Acids & Salts
Example:
HClO = hypochlorous acid
HClO
2
HClO
3
HClO
4
= chlorous acid
= chloric acid
= perchloric
Naming of Acids & Salts
Ternary Salts: Prefix & Suffix
X could be any element other than (Sulfur,
Phosphorus, Nitrogen or Carbon)
XO = hypo-________-ite
XO
2
-
XO
3
-
XO
4
-
= __________-ite
= __________-ate
= per-_________-ate
Naming of Acids & Salts
Example; Chlorine (X represents any metal)
XClO = hypochlorite
XClO
2
-
XClO
3
-
XClO
4
-
= chlorite
= chlorate
= perchlorate