mrs. grindle's acid and molecules nomenclature ppt
advertisement

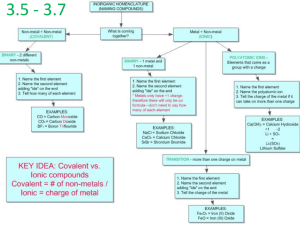
Naming acids covalent/molecular compounds Ch. 2 in book Rules for naming acids 1. Binary Acid (like a simple ionic cpd) Prefix hydro + nonmetal root + suffix -ic + (separate word) acid Ex) HCl = hydro + chlor + ic + acid = hydrochloric acid 2. Oxoacid (like naming with oxoanions but…) -ate becomes -ic in the acid -ite becomes -ous in acid Ex) HBrO4 is perbromic acid Acids practice HBr HIO3 HCN HNO3 Hydrobromic acid Iodic acid Hydrocyanic acid Nitric acid Rules for naming molecules Two nonmetals: 1. Lower group number 1st-except oxygen and halogen-halogen 1st 2. If in same group higher period 1st 3. 2nd element named root with -ide 4. USE GREEK! Greek prefixes for how much of each compound- 1st number only needs if +1 Greek prefixes 1-mono 2-di 3-tri 4-tetra 5-penta 6-hexa 7-hepta 8-octa 9-nona 10-deca Molecules practice PCl5 Formula for carbon disulfide Name and formula for cpd with 2-N and 4-O Phosphorus pentachloride CS2 Dinitrogen tetraoxide – N2O4 If there is a hydrogen in the middle… Example) NaHCO3 How would you name it otherwise? – Ionic: m/PAI Same but add hydrogen (use prefix if +1) Name: sodium hydrogen carbonate Practice: KH2PO4 Potassium dihydrogen phosphate