2.2 Names and Formulas for Ionic and Molecular Formulas web
advertisement

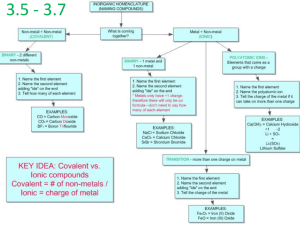
The Formation of Ionic and Covalent Bonds (2.2) Standardized Naming ▪ IUPAC (International Union for Pure and Applied Chemistry) Naming Binary Ionic Compounds ▪ two different elements Rules: 1. Name the metal ion 2. If the metal is a transition metal having more than one charge, indicate the charge with a roman numeral in brackets 3. Name the non-metal ion, add an “ide” ending Example: Naming Ionic Compounds with Polyatomic Ions ▪ treat the polyatomic ion as a single unit ▪ Table 2.4 ▪ do not change the name of the polyatomic ion Example: Writing Chemical Formulas for Ionic Compounds Rules: 1. Write the symbols for the ions (+ve first, -ve second) 2. Determine the charges of the ions (write above the symbol) 3. Crisscross the charges to make subscripts (never write 1, reduce if possible, polyatomic ion subscripts need to go after brackets) Example: Writing Names and Formulas for Acids and Bases ▪ acids ionize (break apart) in water to produce H+ ▪ bases ionize in water to produce OHBases: ▪ same as ionic compounds ▪ (s) means it’s pure form, (aq) means it’s mixed in water ▪ alkali: a base that is soluble in water Naming and Writing Formulas for Acids ▪ named as ionic compounds (even though they are molecular) ▪ current naming (IUPAC) for acids dissolved in water is to add “aqueous” to the start of the name ▪ “old” naming is still used for many acids - acids not containing oxygen: hydro(root)ic acid (see Table 2.7) - acids containing oxygen: complex (see Table 2.8) Writing Names and Formulas for Binary Molecular Compounds ▪ uses prefixes Rules: 1. name the element with the lower group number first (halogens are always first when combined with oxygen, if both in the same group: higher period number first) 2. second element gets “ide” ending 3. use a prefix for the first element (unless there is only one) 4. use a prefix for the second element (if it’s oxygen, omit the O or A from the prefix) Example: Writing Chemical Formulas for Binary Molecular Compounds *inorganic only* Rules: 1. Write the element symbols (using the same rules as above) 2. Use the prefixes to determine the subscripts Example: Drawing Structural Formulas for Molecular Compounds ▪ makes the Lewis structure simpler ▪ replace bonding pairs with a single line and omit lone pairs (fig 2.22) Example: Classwork: Page 75 #5, 6, 8, 9, 10, 12 More Practice: page 73