Understanding the Covalent Bond
advertisement

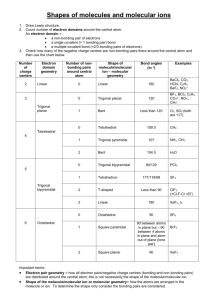
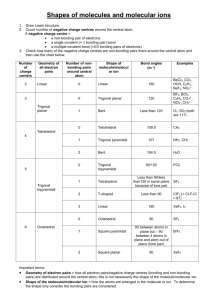
Covalent Bonding: Molecular Geometry Hybridization of Atomic Orbitals Molecular Orbitals 1 2 Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the electron (bonding and nonbonding) pairs. Class # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB2 2 0 linear linear trigonal planar AB3 3 0 trigonal planar AB4 4 0 tetrahedral tetrahedral AB5 5 0 trigonal bipyramidal trigonal bipyramidal AB6 6 0 octahedral octahedral 3 VSEPR linear trigonal bipyramidal tetrahedral trigonal planar octahedral 4 VSEPR Cl Be Cl linear trigonal bipyramidal PCl5 BF3 CH4 tetrahedral trigonal planar octahedral SF6 5 Effects of Lone Pairs H H H C H N H H O H H H lone-pair vs. lone pair lone-pair vs. bonding bonding-pair vs. bonding 6 > > repulsion pair repulsion pair repulsion VSEPR Class # of atoms bonded to central atom # lone pairs on central atom AB3 3 0 AB2E 2 1 Arrangement of electron pairs Molecular Geometry trigonal planar trigonal planar trigonal planar bent 7 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom AB4 4 0 AB3E 3 1 Arrangement of electron pairs Molecular Geometry tetrahedral tetrahedral tetrahedral trigonal pyramidal 8 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom AB4 4 0 Arrangement of electron pairs Molecular Geometry tetrahedral tetrahedral AB3E 3 1 tetrahedral trigonal pyramidal AB2E2 2 2 tetrahedral bent O H H 9 VSEPR Class AB5 AB4E # of atoms bonded to central atom 5 4 # lone pairs on central atom Arrangement of electron pairs Molecular Geometry 0 trigonal bipyramidal trigonal bipyramidal 1 trigonal bipyramidal distorted tetrahedron 10 VSEPR Class AB5 # of atoms bonded to central atom 5 # lone pairs on central atom 0 AB4E 4 1 AB3E2 3 2 Arrangement of electron pairs Molecular Geometry trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal distorted tetrahedron T-shaped F F Cl F 11 VSEPR Class AB5 # of atoms bonded to central atom 5 # lone pairs on central atom 0 AB4E 4 1 AB3E2 3 2 AB2E3 2 3 Arrangement of electron pairs Molecular Geometry trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal distorted tetrahedron trigonal bipyramidal T-shaped linear I I I 12 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom AB6 6 0 octahedral octahedral AB5E 5 1 octahedral square pyramidal F F F Arrangement of electron pairs Molecular Geometry Br F F 13 VSEPR Class # of atoms bonded to central atom # lone pairs on central atom AB6 6 0 octahedral octahedral AB5E 5 1 octahedral AB4E2 4 2 octahedral square pyramidal square planar Arrangement of electron pairs Molecular Geometry F F Xe F F 14 15 Predicting Molecular Geometry 1. Draw Lewis structure for molecule. 2. Count number of lone pairs on the central atom and number of atoms bonded to the central atom. 3. Use VSEPR to predict the geometry of the molecule. What are the molecular geometries of SO2 and SF4? O S AB2E bent F O F S F AB4E F distorted tetrahedron 16 Dipole Moments and Polar Molecules electron poor region electron rich region H F d+ d- m=Qxr Q is the charge r is the distance between charges 1 D = 3.36 x 10-30 C m 17 18 Which of the following molecules have a dipole moment? H2O, CO2, SO2, and CH4 O S dipole moment polar molecule dipole moment polar molecule H O C O no dipole moment nonpolar molecule H C H H no dipole moment nonpolar molecule 19 Does CH2Cl2 have a dipole moment? 20 21 22 Chemistry In Action: Microwave Ovens 23 How does Lewis theory explain the bonds in H2 and F2? Sharing of two electrons between the two atoms. Overlap Of Bond Dissociation Energy Bond Length H2 436.4 kJ/mole 74 pm 2 1s F2 150.6 kJ/mole 142 pm 2 2p Valence bond theory – bonds are formed by sharing of e- from overlapping atomic orbitals. 24 25 Change in electron density as two hydrogen atoms approach each other. 26 Valence Bond Theory and NH3 N – 1s22s22p3 3 H – 1s1 If the bonds form from overlap of 3 2p orbitals on nitrogen with the 1s orbital on each hydrogen atom, what would the molecular geometry of NH3 be? If use the 3 2p orbitals predict 900 Actual H-N-H bond angle is 107.30 27 Hybridization – mixing of two or more atomic orbitals to form a new set of hybrid orbitals. 1. Mix at least 2 nonequivalent atomic orbitals (e.g. s and p). Hybrid orbitals have very different shape from original atomic orbitals. 2. Number of hybrid orbitals is equal to number of pure atomic orbitals used in the hybridization process. 3. Covalent bonds are formed by: a. Overlap of hybrid orbitals with atomic orbitals b. Overlap of hybrid orbitals with other hybrid orbitals 28 Bonding in Methane 29 Fig. 10.7 Formation of sp3 Hybrid Orbitals 30 Formation of sp3 Hybrid Orbitals 31 Formation of a CH4 Molecule 32 Formation of a NH3 Molecule 33 Stylized Drawing of Valence Bond Theory Predict correct bond angle CH4 NH3 Sigma bond (s) – electron density between the 2 atoms 34 Formation of sp2 Hybrid Orbitals 35 Formation of sp2 Hybrid Orbitals 36 Formation of sp2 Hybrid Orbitals 37 2pz orbital is perpendicular to the plane of hybridized orbitals sp2 Hybridization of a C atom 38 Bonding in Ethylene C2H4 H H C H C H Sigma bond (s) – electron density between the 2 atoms 39 Bonding in Ethylene C2H4 Pi bond (p) – electron density above and below plane of nuclei of the bonding atoms 40 Bonding in Ethylene C2H4 41 Formation of sp Hybrid Orbitals 42 Formation of sp Hybrid Orbitals 43 Formation of sp Hybrid Orbitals 44 Bonding in acetylene C 2H 2 45 How do I predict the hybridization of the central atom? Count the number of lone pairs AND the number of atoms bonded to the central atom # of Lone Pairs + # of Bonded Atoms Hybridization Examples 2 sp BeCl2 3 sp2 BF3 4 sp3 CH4, NH3, H2O 5 sp3d PCl5 6 sp3d2 SF6 46 47 Sigma (s) and Pi Bonds (p) 1 sigma bond Single bond Double bond 1 sigma bond and 1 pi bond Triple bond 1 sigma bond and 2 pi bonds How many s and p bonds are in the acetic acid (vinegar) molecule CH3COOH? H C H O H C O H s bonds = 6 + 1 = 7 p bonds = 1 48 Drawback of Valence Bond Theory Experiments show O2 is paramagnetic O O No unpaired eShould be diamagnetic Molecular orbital theory – bonds are formed from interaction of atomic orbitals to form molecular orbitals. 49 An analogy between light waves and atomic wave functions Amplitudes of wave functions added Amplitudes of wave functions subtracted. 50 Energy levels of bonding and antibonding molecular orbitals in hydrogen (H2) A bonding molecular orbital has lower energy and greater stability than the atomic orbitals from which it was formed. An antibonding molecular orbital has higher energy and lower stability than the atomic orbitals from which it was 51 formed. Energy levels of bonding and antibonding molecular orbitals in boron (B2) 52 53 Second-Period Homonuclear Diatomic Molecules 54 Molecular Orbital (MO) Configurations 1. The number of molecular orbitals (MOs) formed is always equal to the number of atomic orbitals combined. 2. The more stable the bonding MO, the less stable the corresponding antibonding MO. 3. The filling of MOs proceeds from low to high energies. 4. Each MO can accommodate up to two electrons. 5. Use Hund’s rule when adding electrons to MOs of the same energy. 6. The number of electrons in the MOs is equal to the sum of all the electrons on the bonding atoms. 55 Bond Order 1 bond order = 2 bond order ½ ( Number of electrons in bonding MOs - 1 ½ Number of electrons in antibonding MOs ) 0 56 MO for 2nd Period Homonuclear Diatomic Molecules MO theory predicts that O2 is paramagnetic! 57 Molecules with Resonance Structures 58 Delocalized π Molecular Orbitals Delocalized molecular orbitals are not confined between two adjacent bonding atoms, but actually extend over three or more atoms. 59 Electron density above and below the plane of the benzene molecule. 60 61 Acknowledgment Some images, animation, and material have been taken from the following sources: Chemistry, Zumdahl, Steven S.; Zumdahl, Susan A.; Houghton Mifflin Co., 6th Ed., 2003; supplements for the instructor General Chemistry: The Essential Concepts, Chang, Raymon; McGraw-Hill Co. Inc., 4th Ed., 2005; supplements for the instructor Principles of General Chemistry, Silberberg, Martin; McGraw-Hill Co. Inc., 1st Ed., 2006; supplements for the instructor 62