8 Molecular Geometry (rev 4.28.10)
advertisement

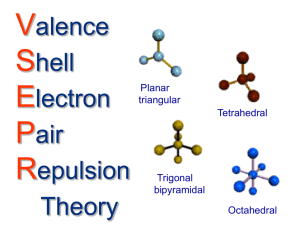
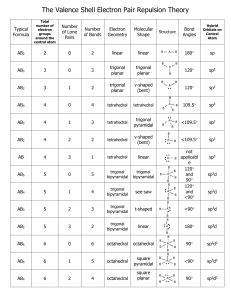
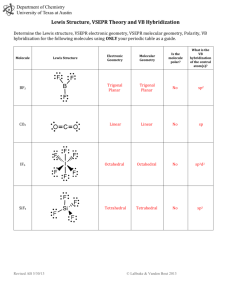
Molecular Geometry - Hybridization VSEPR - Valence shell electron pair repulsion (electron pairs will go as far apart in space as they can) 1. Draw Lewis Structure 2. Count number of electron groups around central atom (a multiple bond is “one” electron group) E 3. Choose electron group geometry based on 2,3,4,5 or 6 groups around the central atom 4. For molecular geometry look only at where the atoms are placed, lone pairs are “invisible” 5. For hybridization, count the number of lone pairs and sigma bonds to find out number of molecular orbitals needed. Count only 1 bond from a double or triple bond as sigma. 2 orbitals = sp 3 orbitals = sp2 4 orbitals = sp3 5 orbitals = sp3d 6 orbitals = sp3d2 6. For 3D drawing, use Lewis structure and place atoms and lone pairs in the locations around central atom appropriately. For 5 trigonal bipyramidal, lone pairs go on corners of triangle (equatorial positions). For 6 octahedral, lone pairs go above and below the plane (axial positions) and not on the 4 corners of the middle square. # e- groups around central atom e- group geometry Example 2 linear 3 Trigonal planar Lewis Structure # lone pairs Geometry designation Molecular shape hybridization CO2 0 AX2 Linear sp BF3 0 AX3 Trigonal planar sp2 NO3- 0 AX3 3 Trigonal planar NO2- 1 AX2E Bent sp2 4 tetrahedral CH4 0 AX4 tetrahedral sp3 3D model 4 tetrahedral NH3 1 AX3E Trigonal pyramidal sp3 4 tetrahedral H2O 2 AX2E2 bent sp3 5 Trigonal bipyramidal PCl5 0 AX5 Trigonal bipyramidal sp3d 5 Trigonal bipyramidal SF4 1 AX4E Seesaw sp3d 5 Trigonal bipyramidal ClF3 2 AX3E2 T-shape sp3d BrF3 5 Trigonal bipyramidal XeF2 3 AX2E3 linear sp3d 6 octahedral SF6 0 AX6 octahedral sp3d2 6 octahedral ClF5 1 AX5E1 Square pyramidal sp3d2 6 octahedral ICl4- 2 AX4E2 Square planar sp3d2 XeF4