lec23_2013 - Andrew.cmu.edu
advertisement

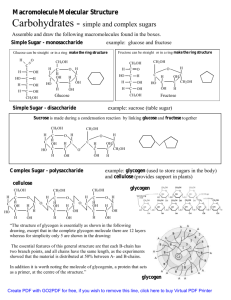
Biochemistry I Spring Term Lecture 23 Carbohydrates I March 18, 2013 Assigned reading in Horton: 8.1-8.4. Nelson 5e: 7.1 Key Terms: aldose ketose epimers hemiacetal anomeric carbon Haworth Representation Carbohydrates are: 1. 2. 3. 4. The primary energy reservoir in biosphere. Biosynthetic precursors to amino acids and nucleic acids. Targeting of proteins for trafficking within the cell. Structural and mechanical components. Cell walls in plants Cell walls in bacteria CH2OH O CH2OH Structural Hierarchy: dihydroxyacetone 1. Monosaccharides: cannot be hydrolyzed to simpler sugars. (no chiral center) 2. Oligosaccharides: 'a few' covalently linked monosaccharides. 3. Polysaccharides: 'many' covalently linked H O O monosaccharides. H Monosaccharides: All carbons in monosaccharides are H OH HO H 'hydrated' -hence the name carbohydrate (general formula CH2OH CH2OH (CH2O)N) S(L) glyceraldehyde D(R) glyceraldehyde 1. The simplest monosaccharides contain three carbons: 2. When the C=O group is at the 2nd position it's called an ketose. 3. When the C=O group is at the very end it's an aldose. 4. Note that the aldose, glyceraldehyde, has a chiral center and therefore exists in D and L forms, or mirror images of each other. The D-form is the "root" compound for all other naturally occurring aldoses. 5. Additional hydrated carbons (HO-C-H) are added just below the aldehyde or ketone group. Therefore, the chiral center of D-glyceraldehyde is preserved. The added carbon generates a new chiral center. The two different molecules generated by the addition of another carbon are called epimers because the differ in only one chiral center. For example, the addition of a CH2OH unit to D-glyceraldehyde gives the following two epimers: erythrose and threose. Aldoses D-Glyceraldehyde Erythrose 1 H O H H H OH OH OH CH2OH H O H H H H OH OH OH OH CH2OH O H H OH OH CH2OH HO H H H H OH OH OH CH2OH H OH CH2OH HO H Threose HO H H O O H H H H H H H HO H H O OH H OH OH CH2OH O H H OH OH CH2OH H HO HO H H H HO H O H OH OH OH CH2OH H H H HO H O H OH CH2OH O H OH H OH CH2OH O OH OH H OH CH2OH H HO H HO H HO HO H O H OH H OH CH2OH H HO HO HO H O H H H OH CH2OH O H H OH CH2OH H H HO HO H O OH H H OH CH2OH Biochemistry I Spring Term Lecture 23 March 18, 2013 Ketoses: CH2OH O CH2OH HO CH2OH O CH2OH dihydroxyacetone H CH2OH CH2OH O H O OH HO CH2OH H H H OH OH CH2OH Important sugars to remember are: Aldose Ketose Glyceraldehyde (C3) [Important in energy metabolism] Fructose (C6) [Important in metabolism] Ribose (C5) [Building block of DNA and RNA] Glucose (C6) [Energy metabolism and structural elements] Ring formation: + H In general, alcohols can attack the C=O group in sugars to form hemiacetals. Since sugars have OH groups, they can form hemiacetals by an intramolecular reaction, forming closed rings. O aldehyde O R2 Only long (>C4) saccharides can form internal hemiacetals, giving closed rings (Includes ribose, glucose, fructose). No atoms are lost or gained in this reaction! H H R2 O R1 H R1 H Ring Formation in Glucose alcohol 1. Six membered ring created by forming a bond between C1 and O5 (most stable ring size). 2. This form is called pyranose, i.e. glucopyranose after the organic compound, pyran. 3. The C1 carbon becomes chiral and is called the anomeric carbon 4. The new OH group (on C1) can exist in either the or form. 5. The and forms can readily inter-convert via the linear intermediate. O Hemiacetal O pyran CH2OH CH2OH H O H H O O OH H HO OH H OH O OH OH OH OH OH HO H OH OH H OH OH CH2OH CH2OH O OH CH2OH H OH CH2OH H OH H O O H OH OH OH OH O H OH 2 Biochemistry I Spring Term Lecture 23 March 18, 2013 C5 Aldose: Ribose 1. Formation of a 5 membered ring can occur by forming a bond between C1 and O4. 2. The cyclic form is called a O furanose (i.e. ribofuranose) after the organic compound, furan. H CH2OH O CH2OH O H O H O OH H OH H OH H O OH CH2OH H O H OH OH OH CH2OH O O CH2OH OH OH H OH OH OH H C6 Ketose: Fructose H Although this is a 6-carbon sugar, because it is a ketose a five membered ring is formed. O OH 1 CH2OH C C This is also called a furanose (i.e. fructo-furanose). HO 3 H 4 C OH C 2 4 2 CH2OH O HO O 3 C O CH2OH CH2OH 5 C OH HO C OH C H OH O H 5 C O CH2OH H OH CH2OH CH2OH O HO 5 C 4 CH2OH C OH C 2 O CH2OH 3 C OH OH How to identify the anomeric carbon: Presentation of structures: Fischer, Haworth, Reduced Haworth Representations. H O H OH HO H H OH H OH CH2OH CH2OH H H HO H OH H OH H H O H OH H H OH H H OH H OH H O H CH2OH OH O OH H OH H OH H CH2OH OH H O OH OH H OH CH2OH OH O OH CH2OH glucose OH H H galactose O OH HO H HO H H OH CH2OH OH O OH CH2OH i) tip clockwise, ii) move –O- to back, iii) down = down, up = up. 3