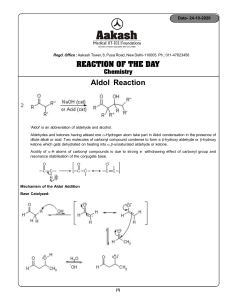
O CH O + 2 eq. NaOH 2
advertisement

Chapter 17 Problem Set 1.(15) In a reaction that my students ran in lab this week, trans cinnamaldehyde reacted with cyclopentanone (in a basic medium) in a double aldol/dehydration reaction to produce a brilliant yellow solid. This product has a formula of C23H20O (although the product only shows 10 carbon resonances in the C-13 NMR spectrum). O O CH + 2 eq. NaOH 2 a) Propose a structure of this yellow compound. O (Reaction does not go through conjugate addition as stated in the original question.) b) Why do you suppose the product requires no acidic dehydration step to lose water (spontaneous dehydration occurs)? The aldol product loses water so readily because the enones that form are conjugated to each other as well as to the aromatic rings – if a product can dehydrate to form a conjugated product in multiple directions, the water will be lost automatically. c) Why do you suppose the compound is yellow and not colorless? The HOMO- LUMO gap is so narrow due to the extensive conjugation, that the λmax falls in the visible portion of the electromagnetic spectrum. 2.(24) a) Draw the C-2 epimer of D-idose. b) Name this carbohydrate. CHO HO H H HO H C-2 H H HO H OH H OH CH2OH CHO OH OH H OH CH2OH D-Idose D-Gulose d) Draw the chair conformation of the β-pyranose form of this sugar. OH CH2OH O OH HO OH d) Can an acetonide be formed between C2 & C3 OH groups? Yes. Between C3 & C4? No. OH groups need to be cis. e) Draw the acetonide that would form upon treatment with one equivalent of acetone and H+. OH CH2OH O OH O CH3 O CH3 f) Draw the α-benzyl glycoside of mannopyranose. OH HO HO CH2OH O OCH2 3.(18) Propose a synthesis of the following compounds from the designated starting materials: For alkylations, it appears best to use LDA as your base. For aldol reactions, it is better to use NaOEt or NaOMe with the alcohol solvent. Equilibrium is close but loss of H2O usually drives the reaction. O O 1) LDA, THF CH3 2) CH3I a) 3) LDA, THF 4) C6H5SeBr 5) H2O2 CH3 6) Li(CH 3)2Cu + 7) H3O 1) O3 b) CHO 2) Zn, HOAc 3) NaOCH3, CH3OH (aldol with loss of H2O) O c) + 1) R2NH, H3O , pH = 4-5 O 2) CH3I 3) NaOEt, EtOH 4) CHO 5) heat (should dehydrate spontaneously) + or 5) H3O