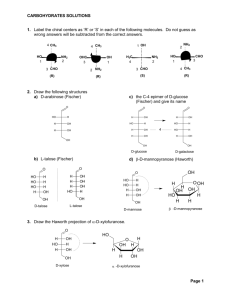
投影片 1
advertisement

Chapter 7 Carbohydrates and Glycobiology Carbohydrates are everywhere http://www.homejoin.com.tw/newpic2000/HST-40.jpg http://www.cofc.edu/~delliss/virtuallabbook/LoadingGel/Minigel2a.jpg http://www.99main.com/~charlief/theeyebg.gif http://sportsdrz.com/images/knee.jpg Sucrose http://www.solarnavigator.net/solar_cola/cola_images/sucrose_table_sugar_crystals.jpg glazed donuts Sucrose (table sugar) http://graphics.stanford.edu/courses/cs348b-competition/cs348b-05/donut/fifthdonut.jpg What are carbohydrates? •Carbo-hydrates are a group of organic compound that can usually represented as (CH2O)n. Glucose, fructose, and galactose are all carbohydrates. a-D-Galactose Classification of carbohydrate –carbon number –L- or D- isomers –pyranose or furanose –α or β anomers –ketose or aldose Carbon number •In addition to simple hexose such as glucose, galactose, and mannose, there are a number of sugar derivatives in which a hydroxyl group in the parent compound is replaced with another substituent, or carbon atom is oxidized to a carboxyl group. •Therefore, researchers must give each carbon of a sugar a number. H H HO H H aldose Carbon number O 1 CH2OH C1 2 H C O C2 OH 3 HO C H C H 3 4 C4 OH H C OH 5 H C OH C5 OH 6 CH2OH CH2OH ketose 6 L- and D- isomers [a]25D=+8.7˚ [a]25D=-8.7˚ CHO | H -C- OH | CH2OH CHO | HO -C- H | CH2OH D -glyceraldehyde L -glyceraldehyde L- and D-isomers •Although L- and D-isomers of glyceraldehydes are truly levorotatory (-) and dextro-rotatory (+), L- and D- sugars are NOT. For example, L-arabinose [a]20D=+105.1˚ and Dfructose [a]20D=-92˚ a-L-arabinose (pyranose form) How to determine L- and D•First, find the chiral carbon C H- C =O | H- C -OH | H- C -H | OH How to determine L- and D•If there is more than one chiral carbon, then… H-C =O | H-C-OH | H-C-OH | H2-C-OH D- H2-C-OH | C=O | H-C-OH | HO- C-H | LH2-C-OH Formation of the cyclic form H O 6 CH2OH 1C 5C O HOH H H C OH C OH H C1 HO C H C C HOH HO C H OH H OH 5 H C OH anomer CH2OH a b Other sugar generate 5-member ring CH2OH 2C O HO C H H C OH 5 H C OH CH2OH 6 HOCH2 O CH OH2OH 5 C H HO C2 C C CH OH2OH H OH H a b anomer (few) The importance of a sialic acid a sialic acid Sialidase (neuraminidase) Normal protein Asialoglycoprotein: Will be removed by asialoglycoprotein receptors in the liver Monosaccharides •Colorless, crystalline solids •Water soluble but not soluble in nonpolar solvent •Taste sweet Monosaccharides have reducing powers http://www.uni-regensburg.de/Fakultaeten/nat_Fak_IV/Organische_Chemie/Didaktik/Keusch/Grafik/fehling2.gif Disaccharide is made by joining two monosaccharides Lactose is only present in milk Sucrose is also called table sugar Trehalose is a major constituent of insect hemolymph 3 ≦oligosaccharide ≦20 Polysaccharides Polysaccharides can serve as fuels, structural elements, and extracellular support. Polysaccharides •Homopolysaccharides –Starch (amylose, amylopectin) –Glycogen –Chitin •Heteropolysaccharides –Peptidoglycan –Agar (agarose, agaropectin) –Glycosaminoglycans Starch and glycogen are polymers of a-D-glucose •The main chain of starch and glycogen are consisted of a-D-glucose joined by (a14) glycosidic bonds. The branch point of starch and glycogen : (a16) •Amylopectin and glycogen have branchs. Cellulose is a polymer of b-Dglucose amylose cellulose Chitin : polymer of b-N-acetylD-glucosamine GlcNAc GlcNAc GlcNAc GlcNAc Peptidoglycan contains heteropolysaccharides Agarose is very important in molecular biology applications http://www.cofc.edu/~delliss/virtuallabbook/LoadingGel/Minigel2a.jpg Glycosaminoglycans are heteropolysaccharides •N-acetylglucosamine or Nacetyllgalactosamine + uronic acid (Dglucuronic or Liduronic acid) Heparin is a natural anticoagulant Glycoconjugates •Proteoglycans – glycosaminoglycan chains covalently joined to a membrane or secreted protein •Glycoproteins – complex oligosaccharides covalently joined to a protein •Glycolipids – membrane lipids with oligosaccharides as hydrophilic head Proteoglycans Site of attachment Trisaccharide bridge Point of attachment Proteoglycan aggregates Glycoproteins •Glycoproteins are carbohydrateprotein conjugates in which the carbohydrate moieties are smaller and more structurally diverse than the glycosaminoglycans of proteoglycans. •Anomeric carbon of carbohydrate + -OH of Ser/Thr or –NH2 of Asn Ser1, Gly5 M Glycophorin A •Glycophorin A has 16 oligosaccharides covalently attached to it, with total 60 to 70 monosaccharide residues. •It is the MN antigen of human erythrocytes. O- and N-linked glycosidic linkages Glycolipid and lipopolysaccharides are membrane components Glycobiology Carbohydrates can be served as informational molecules Sugar serves as “aging” marker of proteins a sialic acid Sialidase (neuraminidase) Normal protein Asialoglycoprotein: Will be removed by asialoglycoprotein receptors in the liver Lectins •Lectins are proteins that bind carbohydrates with high affinity and specificity. Membrane lectins of H. pylori binds to membrane glycoprotein of gastric epithelial cell Membrane lectins (P-, E- and L-selectins) play important role in the movement of immune cells