practice problems
advertisement

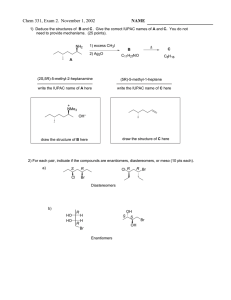
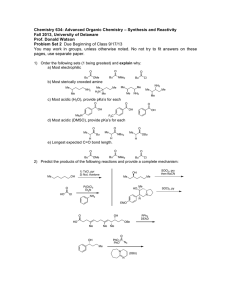
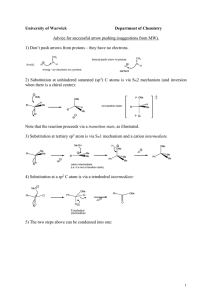
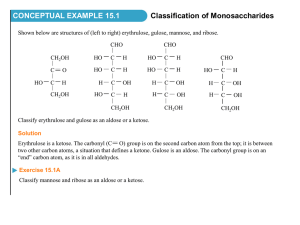
Chem 242 Final Exam Practice Summer 2010 1. Draw the product of each reaction below, and give the reaction mechanism (SN1, SN2, E1, E2). Mechanism CH3OH + I Br + OTs CH3S - + acetone :C N CH3CN Br + EtO - Na+ EtOH 2. Reactions of alkenes with halogens: give the expected product in each case. Be sure to think about reaction mechanisms (and resonance structures for the second reaction). CH3 + Br2 CH3 CCl4 uv + NBS CCl4 3. How many separate signals should the following compounds show in the 1H NMR spectrum? PCl2 CH3-O-CH2CH2CH3 O O Br 4. Circle the strongest nucleophile in each pair below. H2O and Me2NH and HOMeOH R-SH Et3P: and and R-OH Et4P+ 5. A compound of molecular formula C5H10O is shown by chemical tests to contain a carbonyl group (C=O). The 1H NMR shows the following signals: doublet 1.3 ppm 6 H’s Propose a structure for this compound. singlet 2.1 ppm 2 H’s multiplet 2.6 ppm 1H 6. Give products for the following reactions (after aqueous workup if needed). O + CH3MgBr Et2O O Li + C6H12 HO O 2 Et-MgCl + R THF OMe O Cl + CH3-MgBr Et2O 7. In the following molecules, circle any parts of the structure that contain conjugated double bonds. OMe O OMe + NO2 8. Outline a two-step procedure for the synthesis of the compound shown, starting with benzene. Give all reagents for each reaction. Cl 9. Show how the following compounds could be synthesized from methane. a. propane b. methyl isopropyl ether c. 1-butene d. methyl acetate 10. Give the major product(s) for the following reactions of substituted aromatic systems. Cl2 + CF3 FeCl3 O + CH3C -Cl + HNO3 AlCl3 cat. H+ HO O + SO3/H2SO4 11. Show how each starting materials could be transformed into the product shown. Give reagents and draw the product for each step. OH OH O OH OH OH CH3 12. Give products for the following reactions of the alcohol shown. OH HBr + CH2Cl2 OH + H2SO4 H2O OH CrO3 + H2O/acetone 13. Draw examples of the following: a. a monosaccharide that is an aldose and a pentose (five carbons) in the open chain form b. a ketose that is a hexose in the cyclic hemiacetal form c. any disaccharide 14. a. Circle the compounds below that could be carbohydrates. Classify each one (monosaccharide vs. disaccharide, pentose vs. hexose, aldose vs. ketose). CHO H HO H OH HO O H OH CH2OH O O OH HO HO CH2OH OH HO OH HO HO O HO OH OH OMe OH