Topic 3: Alkenes
advertisement

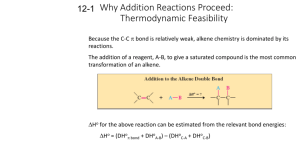
TOPIC 3: ALKENES: REACTIVITY AND SYNTHESIS The chemistry of alkenes is dominated by addition reactions, many of which occur through carbocation intermediates. The information covered in the “Reaction Supplement” is vital to helping you understand, not simply just memorize, the reactions of alkenes. H H H OH First we will cover the general C C C C mechanism of an electrophilic alcohol alkane addition reaction and then look at the regiospecificity of the reaction before we examine X OH C HO C C halohydrin X X C C 1,2-diol C specific alkene addition reactions. OH C alkene C C carbonyl compound 1,2-dihalide H X C C C C halide O C cyclopropane I. ELECTROPHILIC ADDITION REACTION * In this section, we will examine reactions in which an alkene reacts with an electrophile to form a new compound. One of the key factors we will consider is that of regioselectivity, which will help us to determine on which alkene carbon the addition reaction occurs. NUCLEOPHILE: - A “nucleus lover” - Species that donates an electron pair to an electrophile - Nucleophiles are also Lewis bases - Alkenes (the π bond) behave as nucleophiles Remember that the nucleus has a positive charge, so nucleophiles have negative electron density and are in search of a positive charge source ELECTROPHILE: - An “electron lover” - Species that accepts an electron pair from a nucleophile - Electrophiles are also Lewis acids Electrophiles lack electron density and are in search of a negative electron source ELECTROPHILIC ADDITION REACTION: - The addition of an electrophile to an alkene to yield a saturated product - 2 e─ from the π bond form a new σ bond - A carbocation intermediate is formed Reaction Mechanism: H Br Br H3C H3C C H H C H The electrophile HBr is attacked by the nucleophile alkene. A new C – H σ bond & carbocation intermediate form. H3C C H C H3C H The carbocation is now attacked by the Br─ nucleophile. A C – Br bond is formed H Br H3C H3C C H C H The neutral addition product II. ORIENTATION OF ELECTROPHILIC ADDITION: MARKOVNIKOV’S RULE * In this section, we will examine how unsymetrically substituted alkenes give rise to a single addition product even though two possible products can form. We say that such reactions are regiospecific when only one of the two possible orientations occur. REGIOSPECIFIC: - Describes a reaction that occurs when an unsymmetrical reactant yields a specific product (addition at a specific “region”) rather than a mixture of products Cl H3C C H3C A CH2 + B HCl H3C C A H3C H H3C C B Cl H H H H3C C C A B H H 2-chloro-2-methylpropane 1-chloro-2-methylpropane (only product) (NOT formed) MARKOVNIKOV’S RULE: - In the addition of HY to an alkene, the H attaches to the carbon w/ fewer alkyl substituents and the Y attaches to the carbon w/ more alkyl substituents - REASON: In the 1st step of the addition, the C – H bond forms and a carbocation results. If the H adds to the less substituted carbon, the more stable carbocation intermediate will form. Cl H Cl H3C C B H3C C H3C A CH2 + HCl H3C C A H3C H - C A H H3C H C B H H 3o carbocation; 2-chloro-2-methylpropane more stable (only product) B H H3C C A H3C Cl H Cl C B H H - H3C H3C C C A B H H 1o carbocation; 1-chloro-2-methylpropane less stable (NOT formed) III. FORMATION OF HALIDES: ADDITION OF HX TO ALKENES * When HX (X = halogen) is added to an alkene, a halocarbon (halide) forms. This reaction occurs through a carbocation intermediate and follows Markovnikov’s Rule. IV. FORMATION OF 1,2-DIHALIDES: ADDITION OF X2 TO ALKENES * When X2 (X = halogen) is added to an alkene, a dihalide forms. This reaction occurs through a carbocation intermediate. Markovnikov’s Rule does not apply here because addition of X occurs at both alkene carbon atoms. Bromine and chlorine both add readily to alkenes. Fluorine is too reactive to control in most laboratory situations and iodine does not react with most alkenes. H Cl Cl C C H H H 1,2-dichloroethane Approximately 6 million tons per year of 1,2-dichloroethane are synthesized by addition of Cl2 to ethylene. The product is used in the manufacture of polyvinyl chloride (PVC). V. FORMATION OF HALOHYDRINS * When alkenes add HO – Cl or HO – Br under suitable contions, a 1,2-halo alcohol or halohydrin forms. Formation of the halohydrin does not occur by direct addition of HOBr or HOCl, but rather by the addition of Cl2 or Br2 in the presence of water. Because the halogen adds first, the -OH addition site follows Markovnikov’s Rule. HALOHYDRIN: - a “halo alcohol” : - OH and –X groups on adjacent carbons Overview of Reaction: HO Cl2 H2O Cl addition of -OH occurs at the 2o carbon of the alkene Reaction Mechanism: The reaction occurs via addition of X (from X2), followed by addition of H2O, and finally deprotination of water to yield – OH. *See board for mechanism. VI. FORMATION OF ALCOHOLS: ADDITION OF WATER TO ALKENES * Water adds to alkenes to yield alcohols in a process known as hydration. Depending on the reaction conditions used to form the alcohol, either the Markovnikov or anti-Markovnikov product can be selected for. HO H2O H+ OH 1. BH3 2. H2O2, OH- Markovnikov Product anti- Markovnikov Product HYDRATION REACTION: - a reaction in which water is added A. Acid- Catalyzed Alcohol Formation: Markovnikov Product - Reaction of an alkene w/ H2O in the presence of acid to form alcohol - Yields Markonikov product - Occurs via carbocation intermediate (electrophilic addition reaction mechansim) * See board for mechanism B. Hydroboration Alcohol Formation: anti - Markovnikov Product - Reaction of an alkene w/ BH3 (borane) in the presence of H2O2/ OH─ to form alcohol - Yields anti - Markonikov product - Addition of BH3 occurs in a single step via a 4-membered ring where B forms bond to less substituted carbon - BH3 is then substituted by OH ─ to form the alcohol * See board for mechanism VII. FORMATION OF DIOLS: HYDROXYLATION * Hydroxylation of of an alkene occurs when an –OH group adds to each of the alkene carbons. The product is called a diol (short for “di – alcohol”), which is also known as a glycol, a term seen quite often in reference to boimolecules. The reaction does not occur via the traditional electrophilic addition mechanism because no carbocation is formed. C C 1. OsO4 2. NaHSO3 an alkene HO C a diol OH C HYDROXYLATION: - a reaction in which – OH is added to both alkene carbons DIOL: - a dialcohol (2 – OH groups) Alkene hydroxylation does not involve a carbocation intermediate but rather is thought to occur through an intermediate cyclic osmate formed by the addition of OsO4 to the alkene. The cyclic osmate is then cleaved (broken) in a seperate step using aqueous sodium bisulfate, NaHSO3. H3C CH3 CH3 alkene O 1. OsO4 OH 2. NaHSO3 Os O H3C O O H3C cyclic osmate intermediate OH H3C diol VIII. FORMATION OF CARBONYLS: ALKENE CLEAVAGE * In the alkene addition reactions we have seen thus far, the carbon-carbon double bond has been converted to a single bond but the carbon skeleton has remained intact. There are however powerful oxidizing agents that will cleave or break C = C bonds and produce two fragments. O3 C 1 C 2 Zn, H3O+ + C 1 O O C 2 While we won’t go into much detail about the specific mechanism of this reaction, it is important to note that it does not occur via a carbocation intermediate. Rather, it forms a cyclic intermediate similar to that seen for hydroxylation. This reaction is of special interest because it is an example of an organic oxidation/ reduction reaction. Addition of O3 (ozone) forms oxidation product called an ozonide. Oxidation products show an increase in bonds to oxygen. The ozonide, which is explosive and therefore never isolated, is then reduced (reduction in the number of bonds to oxygen) to form the carbonyl compounds. O O3 C C O C oxidation Zn, H3O+ C O step An ozonide reduction step + C O O C