Chapter 4: The Reactions of Alkenes
advertisement

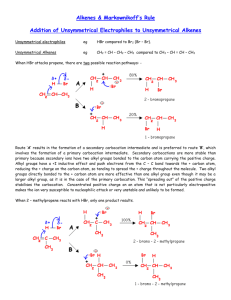
Chemistry 241 Chapter 4: The Reactions of Alkenes Homework: 37, 38a,c, 40a, 42, 44, 45, 46, 47, 48, 50 I. Addition reaction revisited A. Why it happens 1. electrophile (H δ+) and nucleophile (π bond) attraction B. Mechanism 1. 2 steps H Cl a. Cl+ H b. 2. reaction diagram C. Non-symmetrical alkene 1. + H Cl Cl + ? Cl 2. Why? Back to mechanism a. cation formation is slowest step (rds) + more stable/ forms faster than + b. D. Carbocation stability 1. What is 1º, 2º, 3º cation? a. much like amines → how many alkyl substituents 2. relative stability a. methyl < 1º < 2º < 3º 3. why? (hyperconjugation) a. overlap of empty p orbital on cation w/ neighbor sp3 orbital 1 E. Transition state (ts) 1. ts is in between reactant and product (or intermediate) a. top of curve in reaction diagram b. is ts closer to reactant or product 2. Hammond postulate a. ts state is closer in structure to whichever is closer in energy b. since ts highest in energy, closer to reactant or product, which ever of two is higher in energy i. closer to reactant in one step exergonic ii. closer to product in one step endergonic iii. since carbocation intermediate is high in energy, ts closer to carbocation c. therefore, more stable carbocation determines more stable ts d. if both possible carbocations equally stable (both 2º, e.g.), mix of products possible F. Markovnikov’s rule Cl HCl 1. 2. 3. 4. 5. 6. not Cl one constitutional isomer favored over another = regioselective selectivity could be 100% (1º vs 3º) or just major product and minor product (2º vs 3º) or no preference at all (2º vs 2º) Markovnikov’s rule a. the more stable cation determines product b. restated: the side that has more H’s gets the H examples: HBr HBr HBr HBr II. Synthesis strategy A. How to plan for a product 2 HCl ? Cl 1. Cl HCl ? 2. 3. use regioselectivity to find best reactant for your product examples: HCl HCl III. Cl Cl Other acid catalyzed addition reactions A. alkene + HOH → no rxn 1. but add acid catalyst and create much better electrophile H+ (H2SO4) OH + HOH 2. B. Mechanism (3 steps) 1. CANNOT use OH- as base in acidic solution C. Alcohol addition and mechanism 3 OH 1. H2SO4 Example: Draw mechanism for rxn of 1-butene with methanol in sulfuric acid IV. Carbocation rearrangement A. Weird rxns HBr HBr 1. 2. 3. 4. 5. 1,2 Hydride shift 1,2 Methyl shift shift makes carbocation intermediate more stable (and ts) no 1,3 shift ever observed shift only makes carbocation more stable a. 1º → 3º b. 1º → 2º c. 2º → 3º d. not 2º → 2º 6. ring expansion 4 HBr a. b. 3º better than 2º cation AND 5 member ring better than 4 example: V. Addition of halogen to alkene Br2 A. 1. vicinal dibromide or dichloride B. Mechanism 1. Bridged bromonium ion 2. Iodine and fluorine not used C. Format for rxns in organic chemistry other reactants catalyst organic reactant organic product catalyst solvent rxn conditions (heat, etc) D. If water is solvent instead of methylene chloride (CH2Cl2) 1. methylene chloride inert 2. if water used, water is also nucleophile a. not as good as Br-, but a lot greater concentration 3. Halohydrin is result 4. Mechanism 5 5. Other nucleophiles VI. Oxy-mercuration reduction A. Reaction 1. Hg(OAc)2, H2O, THF 2. NaBH4 1. Downside: mercury not very green 2. Upside: no carbocation rearrangement, no acid catalyst 3. mechanism p180 (not required) B. Can also be done in alcohol 1. Hg(O2CCF3)2, methanol 2. NaBH4 VII. Peroxy acid and alkene O R A. O O H + peroxy acid epoxide 1. mechanism p182 (not required) a. concerted (several things happen in one step) 2. Nomenclature of epoxides a. common b. IUPAC 3. Purpose: great oxidizers! More in later chapters VIII. Anti-Markovnikov addition A. Hydroboration Oxidation 1. BH3/THF 2. OH-. H2O2, HOH 1. mechanism p187 (not required) 6 2. great way for anti-Markovnikov addition of alcohol IX. Hydrogenation of alkenes A. Add H to both sides of alkene with metal catalyst H2 Pt/C or Pd/C or Ni B. Can be used to rank stability of alkenes > > > = > > Objectives Knowledge Define new terms: regioselectivity, Hammond postulate, electrophile and nucleophile, electrophilic addition reaction Remember stability order for cations and alkenes (more substituted = more stable) Remember Markovnikov’s rule for addition reactions Comprehension Understand why Markovnikov’s rule is true for those rxns where you have to do the mechanism Use Hammond’s postulate and carbocation stability to explain Markovnikov’s rule Application Given any two of reactant, reaction conditions, and product predict the third for all reactions on p194 and 195 Name simple epoxides W2008 Analysis Draw mechanisms for addition of hydrogen halides, water, and alcohols, vicinal halogenation, and hydrohalogenation reactions Be able to predict hydride and methyl shifts in mechanisms where products can only be explained by these shifts 7