Dehydration of 2-Methylcyclohexanol
advertisement

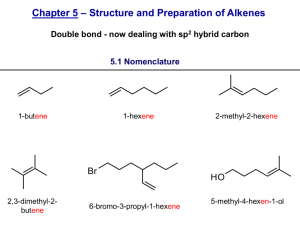
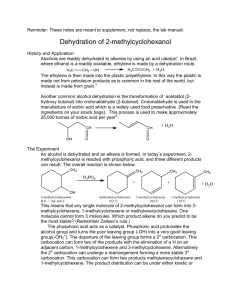
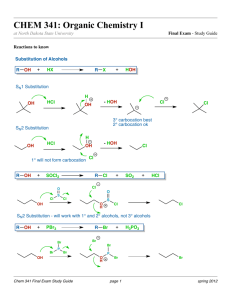
Dehydration of 2-Methylcyclohexanol; Kinetic vs. Thermodynamic Product and Rearrangement References: D. Todd, J.Chem.Ed., 1967, 44(10), 620 (attached) R.L.Taber, W.C.Champion, J. Chem.Ed., 1994, 71(5), 440 (attached) B.W. Moores, Randolph-Macon College http://www.molecules.org/experiments/Moores/Moores.html Penn State, http://courses.chem.psu.edu/chem35/HTML/Experiments/Exp73.pdf Safety Concerns: Wear goggles and gloves the entire time. Phosphoric Acid is very corrosive and will burn skin upon contact. In case of accidental contact wash affected area with copious amount of soap and water. Neutralize any acid spills with sodium bicarbonate. Purpose The purpose of this lab is to a) carry out an alcohol dehydration experiment b) understand Zaitsev’s rule for production of alkenes and c) understand carbocation rearrangements. Chemicals: Name Structure Cis and trans 2methylcylohexanol M.Wt. Density (g/ml) CH3 B.P. (C) 163166 Volume (ml) 10 ml Mass( g) Moles % Yield OH Phosphoric Acid (85%) 1methylcyclohexene 3methylcyclohexene Methylenecyclohex ane 2 H3PO4 CH3 CH3 CH2 Preparation, Set Up and Procedure: 1) Read the reference articles. 2) Fill in missing white cells in above table. Elimination experiment, Revised 11/12/02 slweaver 3) Into a 25 ml Round Bottom flask with stir bar introduce 10 milliliters of 2methylcyclohexanol and 2 ml of 85 % phosphoric Acid. 4) Attach distillation head, thermometer, condenser, distillation vacuum adapter, and graduated cylinder receiving flask. 5) Heat slowly to approximately 96 C. 6) Collect all products, being careful not to let pot boil dry. When approximately 2 ml of liquid remains in round bottom, discontinue heating, measure volume of material collected. 7) Transfer product to a small separatory funnel, wash the organic layer sequentially with 5ml saturated aqueous NaCl, and 5 ml saturated aqueous sodium bicarbonate(NaHCO3). Dry the product over anhydrous sodium sulfate (Na2SO4). 8) Transfer product off of drying agent, record mass. 9) Analyze the product mixture on GC. 10) Based on GC results, fill in shaded cells in above table. GC Parameters OV-1 Column 1 l sample Inj and Det 120oC Column 35 oC Attenuation at 8 Concerns and Potential Discussion Points From the GC trace, how do we know what peak belongs to what component? We obtain a mass of total product (which is a mixture of 3 or 4 materials). How do we determine how much of each component is present? What are the percent yields of each component? Do these observed percent yields agree with what was expected? Why or why not? What was the overall yield? Was this good or not so good? What could have contributed to this yield? Questions: 1. Why doesn’t more phosphoric acid have to be used? 2. The cis and the trans 2-methylcyclohexanol have rates of dehydration that differ by approximately 30. Why? 3. How does the methylenecyclohexane form? 4. Why does methylenecyclohexane form? 5. What product do you expect to be most stable? 6. Why isn’t the most stable product formed exclusively? Elimination experiment, Revised 11/12/02 slweaver Elimination experiment, Revised 11/12/02 slweaver