Electron Configuration and Orbital Diagram
advertisement

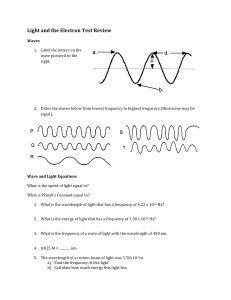
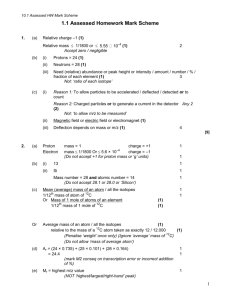
Electron Configuration and Orbital Diagram 1. Which of the following is the correct electron configuration for the bromine, Br? a) [Ar] 4s24p4 b) [Ar] 4s23d104p4 c) [Ar] 4s23d104p5 d) [Ar] 4s23d104p6 e) [Ar] 4s23d103p5 2. Write an orbital diagram for the ground state of the scandium, Sc, atom. How many unpaired electrons does an atom of scandium in the ground state have? 3. Write an orbital diagram for the ground state of the vanadium, V, atom. How many unpaired electrons does an atom of vanadium in the ground state have? 4. Write an orbital diagram for the ground state of the manganese, Mn, atom. How many unpaired electrons does an atom of manganese in the ground state have? 5. For the following electron configuration, decide whether the atom is in the ground state or an excited state. If the configuration is not possible, answer impossible. 1s22s22p23s23p2 6. For the following electron configuration, decide whether the atom is in the ground state or an excited state. If the configuration is not possible, answer impossible. 1s22s22p62d103s1 7. Name the elements whose electron configurations are: 1. 2. 3. 1s2 2s2 2p6 3s2 3p6 4s2 3d3 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d9 1s2 2s2 2p6 3s2 3p6 8. Write electron configurations for these elements: 1. 2. 3. 4. Potassium Lanthanum Copper Bromine 9. Write the electron configuration (ie: 1s2, 2s1), orbital energy diagram, and orbital box diagram for, 1. 2. 3. 4. 5. H, Li, Na, and K Be, Mg and Ca C, Si and Ge F, Cl and Br Fe, Ni and Zn