Uploaded by
lurgreaed
Chemistry Orbitals Worksheet: Electron Configuration & Structure
advertisement

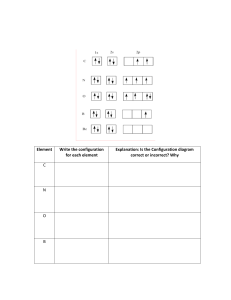
Lurgrea Central College Science Department Chemistry: Orbitals Dr. Grace Batlle Name_______________________________ I. Identify the orbitals ( identifica los orbitales) ____________ II. Date_____________________ __________ _________ _________ Match (pareo) 1. Orbital S a. They are at level 1 and charge up to 2 electrons 2. Valence electrons b. An atom can have 7 levels 3. Orbital F c. They are at level 3 and charge up to 10 electrons 4. Orbital D d. electrons found in the outermost energy shell 5. Energy Levels (niveles de energía) f. They are at level 4 and charge up to 14 electrons 6. Orbital P g. They are at level 2 and charge up to 6 electrons 7. Orbital s h. S P D F III. The following electron configurations belong to which elements: a) 1s2 2s2 2p6 3s1 ______________ b) 1s2 2s2 2p6 3s2 3p6 4s2 3d6 _______________ c) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 ____________ d) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2___________ e) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2 5f5 _____ IV. V. Draw the Bohr models for the following elements and found the valence electrons E.V________ EV______ VI. Fill in the Lewis Dot Structure for each of the elements EV_______