Lesson 3- Cyclic and Aromatic Hydrocarbons - fm-orgchem-2012
advertisement
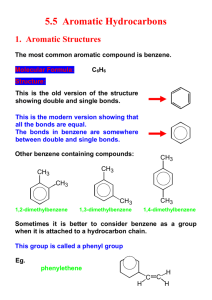
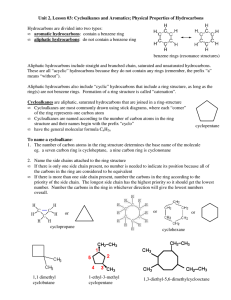
Cyclic and Aromatic Hydrocarbons SCH4U Spring 2012 Cyclic Hydrocarbons (Cyclo-) • • • • Hydrocarbon chains that form rings Have at least three carbon atoms Can be alkanes, alkenes and alkynes General formula for cyclic alkanes: CnH2n Naming cyclic hydrocarbons • Identify the root o Preceded by “cyclo-” • Identify the suffix o Not necessary to indicate the location of the double or triple bonds • Identify the prefix o If there are no or only one side group on a cyclic alkane, the carbon atoms are not numbered o If there are two or more side groups on a cyclic alkane, numbering must give the lowest possible numbers to all side groups o If the molecule is a cyclic alkene or cyclic alkyne, the multiple bond takes highest priority • Name the compound Questions • Name the following: Questions • Draw the following: o 4-ethyl-2-methylcyclopentene o methylcyclobutane o 2-ethyl-3-propylcyclohexane o 1, 2-dimethylcyclohexane Aromatic hydrocarbons (-benzene) • Derived from benzene ring (C6H6) • Aliphatic compounds: do not contain a benzene ring • Benzene ring: o All six carbon-carbon bonds are identical in length o Length of the carbon-carbon bonds is intermediate between a single and a double bond o Resonance hybrid o Delocalized electrons: electrons that make up the second bond in the “double bonds” are equally shared by all six carbon atoms o Conjugated double bonds o Stable structures Naming aromatic hydrocarbons • Identify the root (-benzene) • Identify the prefix o prioritize alkyl side groups with six or fewer carbon atoms in alphabetical order o Continue to number in the direction of the nearest side group • Name the compound (prefix+root) Naming aromatic hydrocarbons • If a benzene ring is attached to a single hydrocarbon chain that has more than six carbon atoms, the benzene ring becomes a side group (-phenyl) Questions • Name each aromatic hydrocarbon: Drawing aromatic hydrocarbons • Draw the benzene ring • If there is more than one side group, number the carbon atoms in the ring • Add the side groups (have six or fewer carbon atoms) Questions • Draw the condensed structural formula for each aromatic hydrocarbon: o 1,3-diethyl-4-methyl-2-propylbenzene o 1-ethyl-3-propylbenzene • Draw the line structural formula for: o 2-phenyl-5-propyloctane o 1,4-dimethylbenzene