Hydrocarbons are divided into two types: aromatic hydrocarbons
advertisement
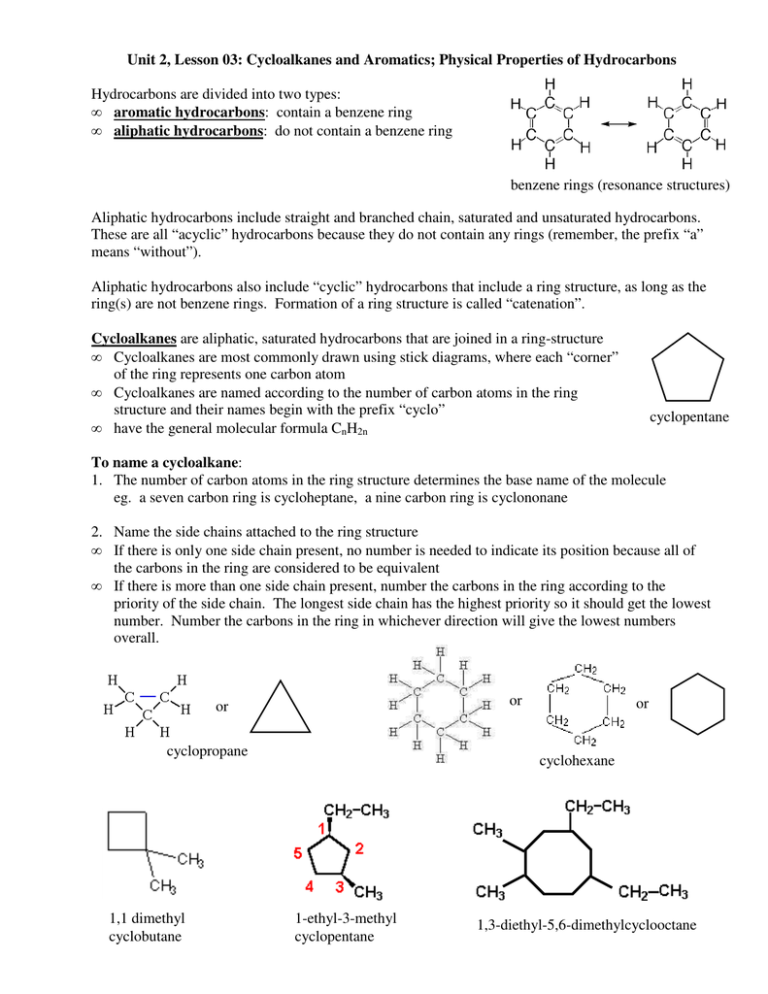
Unit 2, Lesson 03: Cycloalkanes and Aromatics; Physical Properties of Hydrocarbons Hydrocarbons are divided into two types: • aromatic hydrocarbons: contain a benzene ring • aliphatic hydrocarbons: do not contain a benzene ring benzene rings (resonance structures) Aliphatic hydrocarbons include straight and branched chain, saturated and unsaturated hydrocarbons. These are all “acyclic” hydrocarbons because they do not contain any rings (remember, the prefix “a” means “without”). Aliphatic hydrocarbons also include “cyclic” hydrocarbons that include a ring structure, as long as the ring(s) are not benzene rings. Formation of a ring structure is called “catenation”. Cycloalkanes are aliphatic, saturated hydrocarbons that are joined in a ring-structure • Cycloalkanes are most commonly drawn using stick diagrams, where each “corner” of the ring represents one carbon atom • Cycloalkanes are named according to the number of carbon atoms in the ring structure and their names begin with the prefix “cyclo” • have the general molecular formula CnH2n cyclopentane To name a cycloalkane: 1. The number of carbon atoms in the ring structure determines the base name of the molecule eg. a seven carbon ring is cycloheptane, a nine carbon ring is cyclononane 2. Name the side chains attached to the ring structure • If there is only one side chain present, no number is needed to indicate its position because all of the carbons in the ring are considered to be equivalent • If there is more than one side chain present, number the carbons in the ring according to the priority of the side chain. The longest side chain has the highest priority so it should get the lowest number. Number the carbons in the ring in whichever direction will give the lowest numbers overall. or or cyclopropane 1,1 dimethyl cyclobutane or cyclohexane 1-ethyl-3-methyl cyclopentane 1,3-diethyl-5,6-dimethylcyclooctane Cycloalkenes are hydrocarbons that are joined in a ring-structure and contain at least one double bond, but are not benzene rings • Cycloalkenes are named according to the number of carbon atoms in the ring structure and are named “cyclo___ene” • The double bond has naming priority, so the carbon with the double bond is ALWAYS assigned position number 1. Because it is always on C-1, when there is only one double bond, the position number does not need to be included in the name • The positions of any side chains are indicated relative to the double bond (which is C –1). Number the carbon atoms in the ring in whichever direction will give the side chains the lowest position numbers overall • Cycloalkenes with two double bonds are named “cyclo_____diene”. Position numbers for the double bonds must be given if a cycloalkene has more than one double bond or or cyclohexene 4-ethyl-3,6-dimethylcyclohexene 3,4-dimethylcyclopentene 2-ethyl-3-methyl-1,3cyclooctadiene Aromatic Hydrocarbons are organic molecules that contain a “benzene” ring structure • benzene compounds often have odours or “aromas”, which is why they are called “aromatic” • benzene is a six-carbon ring that contains three double bonds • its chemical formula is C6H6. • because of the double bonds, it forms a resonance structure with the electrons in the double bonds moving rapidly in an “electron cloud” above and below the molecule • just as with other resonance structures (Unit 1) the bond length between the carbon atoms is halfway between the length of a single and double bond • the molecule is flat (planar) and it is an extremely stable structure • the benzene ring can be represented many ways: or or the circle represents the “electron The hydrogen atoms on the benzene ring can be replaced with other functional groups, including cloud” resonance structures alkyl groups, hydroxyl groups, halogens and many others. methylbenzene (common name: toluene) hydroxylbenzene (common name: phenol) 2,4,6-trinitromethylbenzene (a.k.a. trinitrotoluene, TNT) To name an aromatic compound using the IUPAC system, follow the same rules as for cycloalkanes, but use benzene as the base name. eg. • • • eg. • • • • this is an aromatic hydrocarbon, base name benzene there are two side chains, the ethyl group is longer so it takes precedence in naming (start numbering with it) name is 1-ethyl-2-methylbenzene this is an aromatic hydrocarbon, base name benzene there are two side chains, both side chains are propyl groups name is 1,3-dipropylbenzene this molecule could also be named meta-dipropylbenzene (see below) When there are two side chains attached to a benzene ring, the position of the side chains relative to one another can be indicated either using position numbers or using an older system: 1. If the side chains are attached to adjacent carbon atoms, then the prefix “ortho” can be used 2. If the side chains are attached to carbons 1 and 3, then the prefix “meta” can be used 3. If the side chains are attached directly across from one another (carbons 1 and 4), then the prefix “para” can be used. ortho-dimethylbenzene or !,2-dimethylbenzene meta-dimethylbenzene or !,2-dimethylbenzene para-dimethylbenzene or !,2-dimethylbenzene ortho-diethylbenzene (1,2-diethylbenzene) Physical Properties of Hydrocarbons Hydrocarbons contain only carbon and hydrogen • the ∆EN of the C – H bond is 0.35, which is non-polar • the ∆EN of the C – C bond is 0.00, also non-polar • because hydrocarbons contain no polar bonds, and no lone electron pairs, they are all non-polar molecules (regardless of the symmetry of the molecules) Because all hydrocarbons are non-polar molecules: • the only type of inter-molecular attraction is London dispersion forces, which are very weak • they are insoluble in water (which is polar) • they are soluble in other non-polar substances • they have very low melting and boiling points, but the melting and boiling points depend on the length of the carbon chain and whether or not the hydrocarbon is branched or unsaturated 1. Effect of Chain Length on Melting and Boiling Points of Hydrocarbons • in general, as the length of the carbon chain increases, the opportunity for London dispersion forces increases, so melting and boiling points also increase (see graph) • C1-C4 alkanes are gases • C5-C16 alkanes are liquids • C16-C24 are solids Melting and Boiling Points for Straight Chain Alkanes vs. Number of Carbon Atoms 2. Effect of Branching on Melting and Boiling Points • straight chain (unbranched) hydrocarbons have stronger dispersion forces than for branched molecules. This is because straight chains can line up beside one another which allows stronger London dispersion forces • branched hydrocarbons have weaker dispersion forces which results in lower melting points and lower densities than the straight chain molecules with the same number of carbons 3. Effect of Unsaturation on Melting and Boiling Points • double bonds tend to make the carbon chain “kinky”- the double bond introduces a bend in the molecule • because of the “kinks”, alkene molecules do not pack together as well as alkanes, so they have lower London dispersion forces • this results in alkenes having lower melting and boiling points that their corresponding alkanes In summary, all hydrocarbons are non-polar covalent. This results in the following properties: 1. low melting and boiling points 2. they are often gases (C1 – C3), liquids (C4 – C16) or very soft solids (C17 +) at SATP 3. they are insoluble in water, which is a polar solvent (If a liquid does not dissolve in a liquid, the liquids are said to be immiscible. If a solid does not dissolve in liquid, it is said to be insoluble.)





![LC Fuels and Thermochemistry [PDF Document]](http://s3.studylib.net/store/data/008241147_1-6ebc9d449a7896c353ddca434fe5df53-300x300.png)



