Aromatic Hydrocarbons
advertisement
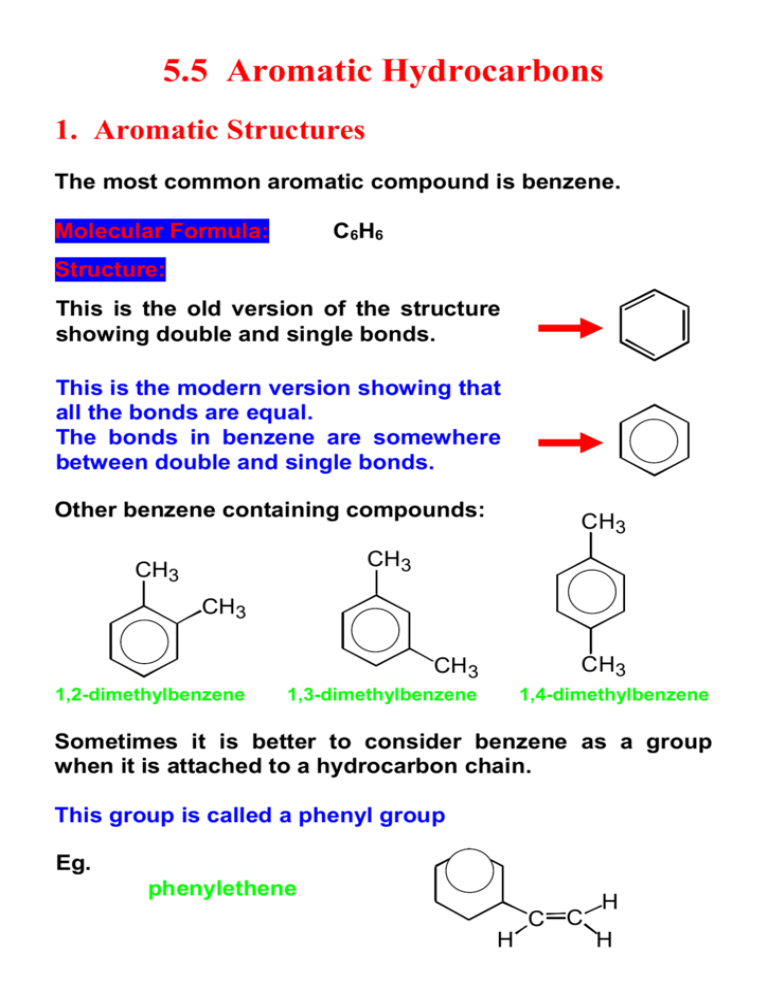
5.5 Aromatic Hydrocarbons 1. Aromatic Structures The most common aromatic compound is benzene. Molecular Formula: C6H6 Structure: This is the old version of the structure showing double and single bonds. This is the modern version showing that all the bonds are equal. The bonds in benzene are somewhere between double and single bonds. Other benzene containing compounds: CH3 CH3 CH3 CH3 CH3 CH3 1,2-dimethylbenzene 1,3-dimethylbenzene 1,4-dimethylbenzene Sometimes it is better to consider benzene as a group when it is attached to a hydrocarbon chain. This group is called a phenyl group Eg. phenylethene C C H H H 2. Aromatic Properties This activity looks at some of the properties methylbenzene (toluene) as an example of a typical aromatic hydrocarbon. Property Observation Appearance Colourless liquid Solubility Insoluble pH Neutral Combustion Burns with smoky flame Bromine Test No reaction Like other unsaturated hydrocarbons aromatic compounds burn with a smoky flame. The bonds in aromatic hydrocarbons are not true double bonds and this is why the bromine does not decolourise. In fact, the benzene ring in aromatic compounds is quite stable. As a result, the benzene ring in aromatic hydrocarbons is resistant to addition reactions. 3. Products from Aromatic Hydrocarbons This activity considers the uses of aromatic hydrocarbons. petrol crude oil naphtha Pain killers Explosives Detergents Agrochemicals Dyestuffs Plastics & fibres Example: (explosives) CH3 O2N NO2 NO2 2,4,6-trinitrotoluene (TNT) Aromatic hydrocarbons are extracted from the naphtha fraction of crude oil. These aromatic compounds can be blended with the petrol fraction of crude oil to give smoother burning fuels or used to make consumer products. As a result, there is a competing demand for the naphtha fraction of crude oil.











