Hydrocarbon Rings
advertisement

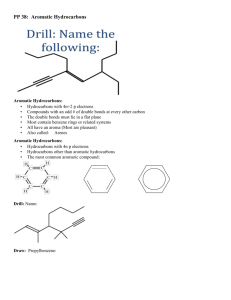
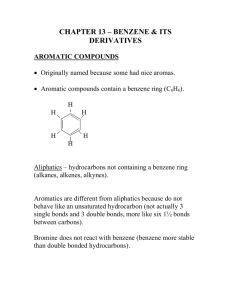
Hydrocarbon Rings Chemistry 122/121 Cyclic Hydrocarbons Both saturated and unsaturated hydrocarbons may be found in the form of a ring The resulting structure has two less hydrogen than its straight chain counterpart ◦ Butane = C4H10 ◦ Cyclobutane = C4H8 Aromatic Hydrocarbons Historically, these compounds all had an odor associated with them They may contain a single ring or group of rings Benzene is the simplest aromatic ◦ all examples of aromatics have a structure comparable to benzene Another name for them are arenes The Structure of Benzene Benzene is a six carbon ring with a hydrogen atom attached to each carbon This leaves each carbon available to make a double bond A maximum of three double bonds can form within the ring As a result, two diagrams can be written to represent all the places a double bond can exist within benzene Page 710 Resonance As a result of double bonds being found in more than one place, the bonding ebetween carbon atoms are shared evenly around the ring Resonance allows the structure to be more stable Benzene is less reactive than its alkene counterpart Substituted Aromatic Hydrocarbons Compounds that contain substituents attached to a benzene ring are called derivatives of benzene When the benzene ring is the substituent, it is called a phenyl group Benzene rings that have two substituents are called disubstituted benzenes The positions of the substituents can be in the 1, 2 (ortho), 1,3 (meta), or 1,4 (para) position For the remainder of class… Complete Guided Reading 22.4 Practice Problems – use to review Interpreting Graphics – 22.4