Chapter 10-11
advertisement

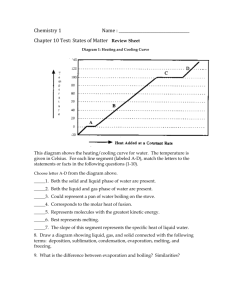
OPRFHS-NGSS Curriculum Alignment Big Idea(s)/ Unifying Q(s): DCI-NGSS SEP-NGSS CCC-NGSS Unit: Chapter 10 States of Matter 11 Gasses Mass, velocity and arrangement of atoms all has an affect on energy 10. Kinetic-Molecular Theory of Gasses Diffusion and Effusion Surface tension Capillary action Crystalline solid Amorphous solid Dynamic Equilibrium Equilibrium Vapor Pressure DCI, EOB and 1. Chemistry 577 10. Melting, freezing, fusion Evaporation, vaporization, boiling, condensing Sublimation, deposition Specific Heat Heat of fusion (Molar enthalpy of fusion) Heat of vaporization (Molar enthalpy of vaporization) Boiling Point, melting point Normal Boiling Point Critical Pressure 11. Avogadro’s Law Ideal Gas Law Combined Gas Law (and variations) STP Effusion Diffusion Graham’s Law of effusion Kelvins – Deg C conversions Absolute zero Atm, mm Hg, kPa conversions 11. Student Learning Targets (coded to DCI, EO or OPRF Objectives) I can calculate the energy flow into our out of a substance using the q=mct equation. I can calculate the specific heat of a substance. I can calculate the energy flow into or out of a system using the molar enthalpy of vaporization or the molar enthalpy of fusion. I can identify phase changes and energy flow and/or identify the phases present given a heating or cooling curve. I can identify phase changes and/or identify the phases present given a phase diagram and pressure/temperature conditions. I can explain and apply the kinetic molecular theory to laboratory observations of solids, liquids and gasses. I can explain the difference between evaporation and sublimation I can explain how molecules move in solids, liquids and gasses I can identify sublimation, melting and boiling points on triple point graphs as well as heating curves I can explain what happens at the triple point of a phase diagram I can predict boiling points using a vapor pressure graph I can use the PVNRT law to calculate any of the missing variables I can use the combined gas law to solve for any of the missing variables I can convert temperatures between K and deg C I can calculate pressure of gasses using the Law of Partial Pressures I can compensate for the vapor pressure of water when calculating the pressure of a gas collected over water. I can calculate the molar mass of gasses I can calculate the density of gasses I can use Grahams Law of Effusion to calculate relative rates of effusion between different gasses OPRFHS-NGSS Curriculum Alignment Chemistry 577 Classroom Instructional Activity Bank Labs/Lab Activities/Videos/Demonstrations Heating Oil and Water mini-lab (Vinier) Determining the Heat of Fusion of snow (ice) (from flip chart Question) Energy and phase changes of water (Vernier) Dry ice triple point lab page 358 and handout Determining the specific heat of a metal Vapor Pressure dropper/pressure demo PT squeeze bottle demo Gas demonstration day Molar Volume of a Gas Lab Grahams Law of Effusion Lab Alka Seltzer Gas Collection: Subtracting Vapor Pressure of Water Investigations/Engineering Projects: Designing an Air Bag Unit: Chapter 10 States of Matter 11 Gasses Resource Bank Worksheets/Reading Guides/Formative Assessments/On-line Homework Bozeman AP Chem Solids and Liquids (7 min) Bozeman AP Chem Thermodynamics-Temperature (4 minutes) Bozeman AP Chem Heat Exchange (5 min) Khan: States of Matter (19 min) Khan: Specific hat, heat of fusion and vaporization (15 min) Chapter 10 chapter review problems pg 353: 5, 6, 9, 13, 19ab, 20, 21ab, 22ab, 26ab?, 27, 28, 29, 30, 35 Chapter 10 standardized test prep pg 357 5, 8, 9, 10, 11 Geek: AP Chem Review: Phase Diagrams #2 Geek: Chemistry review: Calculations involving Specific Heat and latent heat Geek: AP Grahams Law of Effusion Geek: Gas Laws Geek: PVNRT Problems Study Guide Chapter 10-11 Summative Common Unit Assessment: Chapter 10-11