Molar Heat of Fusion and Vaporization
advertisement

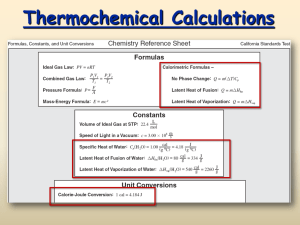
Molar Heat of Fusion and Vaporization DIRECTIONS: Solve the following problems. Show all work in the space provided. Record answers with the correct units and significant figures in the space provided. SHOW WORK HERE RECORD ANSWERS HERE 1. What quantity of heat is released when 506 g of liquid water freezes? 2. The molar heat of fusion for water is 6.009 kJ/mol. Express this heat of fusion in J/g. 3. What mass of steam is required to release 4.97 x 105 kJ of heat energy on condensation? 4. The molar heat of fusion for water is 6.009 kj/mol. How much energy would be required to melt each of the following: a.) 12.75 mol of ice b.) 6.48 x 105 kg of ice 5. Calculate he molar heat of vaporization of a substance given that 0.433 mol of the substance absorbs 36.5 kJ when it is vaporized. Version 1 3/9/2016