CHM 101
advertisement

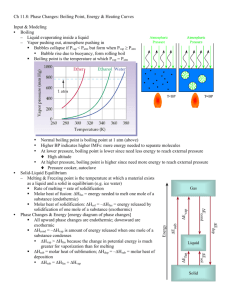
CHM 101 GAGE FALL 1999 NAME __________________________________ SECTION 4225 EXAM 3 SHOW ALL WORK TO RECEIVE CREDIT FOR PROBLEMS 1. Supply the most appropriate word, phrase, or value for each of the following statements: (26) a. term for solid matrix made of repeating unit cells a._________________________ b. standard temperature in kelvins b._________________________ c. crystal type that has high melting point but does not conduct when melted d. general term for a substance that reduces surface tension c._________________________ d._________________________ e. value for standard molar volume e._________________________ f. term for change from a solid to liquid f._________________________ g. intermolecular forces between molecules of bromine g._________________________ h. two factors that affect boiling point h._________________________ ________________________ i. example of a molecular crystal i._________________________ j. two characteristics of a gas based on the Kinetic Molecular Theory j._________________________ _________________________ k. term for shapeless solid 2. k.________________________ What mass of dintrogen oxide gas will occupy 3.8 liters at 980. mmHg and 29oC? 1 (9) 3. Calculate the amount of energy transferred when 225 grams of water change from liquid water at 0OC to 130OC. (15) O sp.ht ice = 2.05 J/g$ C Hvap = 2.25 kJ/g sp.ht liquid = 4.18 J/g$OC sp.ht steam = 2.10 J/g$OC Hfus = 0.330 kJ/g This process is: exothermic endothermic (circle one) 4. A gas sample has a volume of 550 mL at STP. What is its volume at 480 mmHg and 100oC? (9) 5. Complete the following chart. Species (9) Molecular Geometry (name only) Polar or Non-Polar Molecule? NH2F BH3 SeI6 2 Type of Hybridization 6. 7. Put the following sets of compounds in order from lowest to highest boiling point and explain what factor(s) cause them to be in that order. (8) a. CH4 H2O NH3 _________________________________ b. NF3 AsF3 SbF3 _________________________________ Answer any four of the following completely but briefly. You may answer a fifth one for extra credit. If you answer more than 5 you must indicate which ones you wish to be graded or only the first 5 in order will be read. Use the back of this paper to continue. (24) a. Explain why water is a liquid at room temperature while H2S with a higher molar mass is a gas. b. You subject a sample of a gas to high pressure and low temperature. When you measure the volume of the gas it is higher than you predicted it should be. Explain why this might happen. c. Explain why a substance will convert to a gas even though it means going to a higher energy state. d. How can you change the boiling point of a liquid without adding anything? Why does it work? e. Explain what happens when a gas in a rigid container is heated and why. Consider what is happening at the particle level. f. Water is particularly efficient at cooling your body. Explain how and why. g. Sulfur dioxide with a molar mass of 64 g/mol is a gas at room temperature while silicon dioxide with a molar mass of 60 g/mol is a solid with a very high melting point. Explain why. 3