Chem Chp 4 Review
advertisement
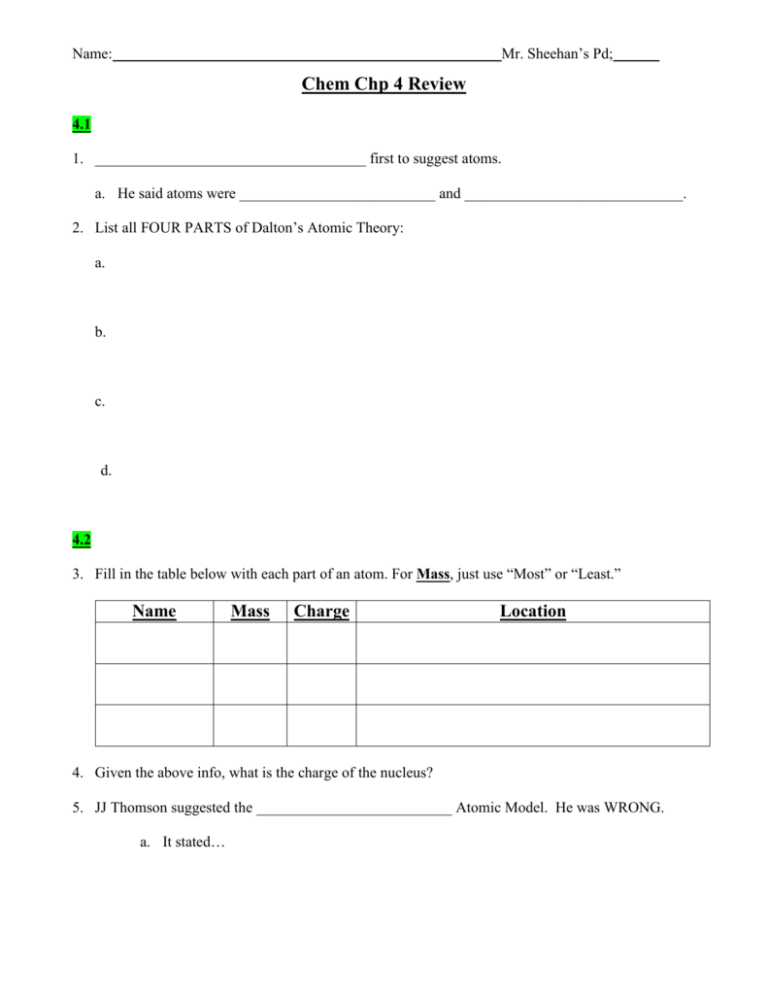
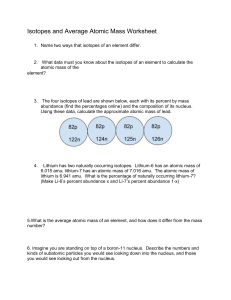
Mr. Sheehan’s Pd; Name: Chem Chp 4 Review 4.1 1. ____________________________________ first to suggest atoms. a. He said atoms were __________________________ and _____________________________. 2. List all FOUR PARTS of Dalton’s Atomic Theory: a. b. c. d. 4.2 3. Fill in the table below with each part of an atom. For Mass, just use “Most” or “Least.” Name Mass Charge Location 4. Given the above info, what is the charge of the nucleus? 5. JJ Thomson suggested the __________________________ Atomic Model. He was WRONG. a. It stated… Mr. Sheehan’s Pd; Name: 6. Rutherford did an experiment where he shot alpha particles at a piece of gold foil, and he came to two conclusions based on his results a. Most particles went right through the foil, proving… b. Rarely, particles were deflected, proving…. c. His model was called the… 4.3 7. Elements are different because they have different numbers of _________________________. 8. What does the ATOMIC number show? 9. What does the MASS number show? Write down as an equation. 10. Complete the table below: 89 25 11. The MASS NUMBER is always ______________________ than the ATOMIC NUMBER. 12. Isotopes are atoms of the ______________________________ with the same number of protons, and different _______________ numbers due to varying number of ________________________. Mr. Sheehan’s Pd; Name: 13. Fill in the table below What are the mass #’s of the following: Given mass #, use hydrogen to write out the isotope below: Lithium-6 1 Lithium-7 2 Lithium-8 3 14. Lithium has two naturally occurring isotopes, Li-6.0 and Li-7.0, their percentage of abundance is 7.5 % and 92.5 % respectively. What is the average atomic mass of Lithium? 15. Element X has three naturally occurring isotopes. The mass (amu) and % abundance of the isotopes are 37.919 amu (5.07%), 39.017 amu (15.35%), and 42.111 amu (79.58%). What is the average atomic mass of element X? 16. A certain element has three isotopes. The isotopic masses and abundances are: 159.37 amu (30.60%), 162.79 amu (15.79%), and 163.92 amu (53.61%). What is the average atomic weight of the element? 17. The Periodic Table arrange elements into ________________ based on repeating _____________. 18. Elements in the same ________________________ have similar properties. 19. Label a PERIOD and a GROUP on the PT below.