Chapter 4 Homework SOLUTION
advertisement
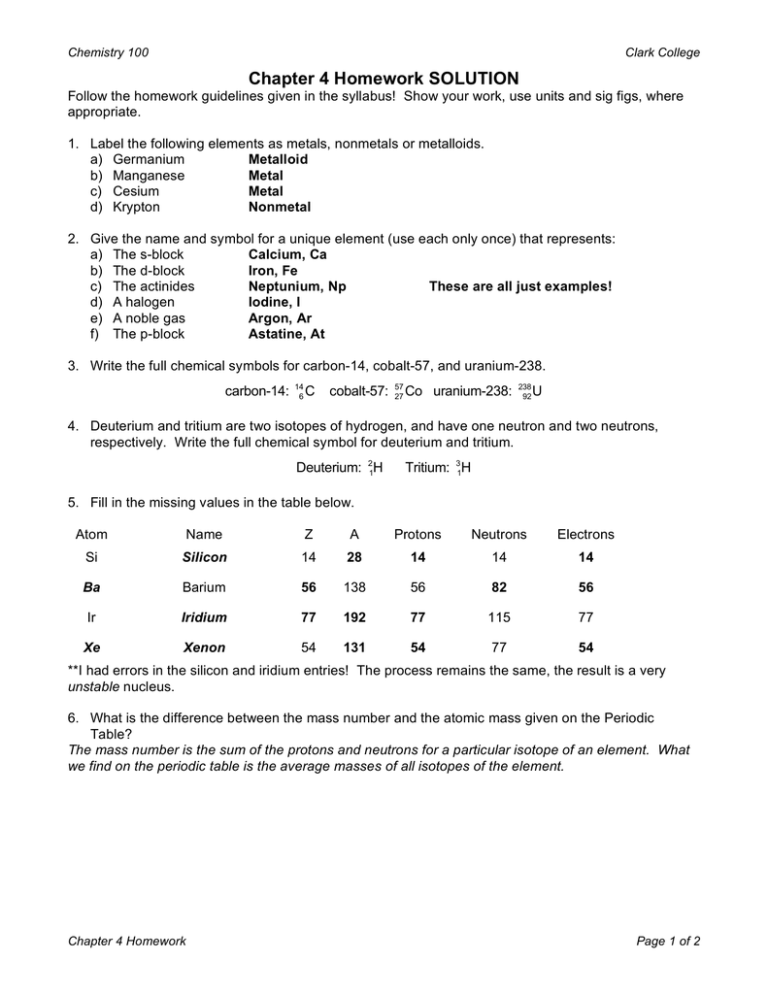
Chemistry 100 Clark College Chapter 4 Homework SOLUTION Follow the homework guidelines given in the syllabus! Show your work, use units and sig figs, where appropriate. 1. Label the following elements as metals, nonmetals or metalloids. a) Germanium Metalloid b) Manganese Metal c) Cesium Metal d) Krypton Nonmetal 2. Give the name and symbol for a unique element (use each only once) that represents: a) The s-block Calcium, Ca b) The d-block Iron, Fe c) The actinides Neptunium, Np These are all just examples! d) A halogen Iodine, I e) A noble gas Argon, Ar f) The p-block Astatine, At 3. Write the full chemical symbols for carbon-14, cobalt-57, and uranium-238. carbon-14: 14 6 C cobalt-57: 57 27 Co uranium-238: 238 92 U 4. Deuterium and tritium are two isotopes of hydrogen, and have one neutron and two neutrons, respectively. Write the full chemical symbol for deuterium and tritium. Deuterium: 21H Tritium: 31H 5. Fill in the missing values in the table below. Atom Name Z A Protons Neutrons Electrons Si Silicon 14 28 14 14 14 Ba Barium 56 138 56 82 56 Ir Iridium 77 192 77 115 77 Xe Xenon 54 131 54 77 54 **I had errors in the silicon and iridium entries! The process remains the same, the result is a very unstable nucleus. 6. What is the difference between the mass number and the atomic mass given on the Periodic Table? The mass number is the sum of the protons and neutrons for a particular isotope of an element. What we find on the periodic table is the average masses of all isotopes of the element. Chapter 4 Homework Page 1 of 2 Chemistry 100 Clark College 7. Calculate the average molar mass for Chromium, Cr, from the isotopic data given. Isotope % Abundance Atomic Mass 50 Cr 4.345 49.946 52 Cr 51.940 83.789 53 Cr 9.501 52.940 54 Cr 2.365 53.939 Avg. Mass = weighted average of the isotopes = [0.04345( 49.946 amu)] + [0.83789(51.940 amu)] + [0.09501(52.940 amu)] + [0.02365(53.939 amu)] = 51.996 amu 8. Only two isotopes of boron (B) are naturally occurring, their relative abundances are given in the table below. If the average atomic mass of boron is 10.811, determine the atomic mass of boron11. Isotope % Abundance Atomic Mass 10 19.61 10.013 11 80.39 ? B B [ ( )] [ ( )] 10.811 = 10.013 amu 0.1961 + x amu 0.8039 x = 11.01 amu 9. Predict the charge for ions from the following elements, based on their positions on the periodic table. a) S, sulfur. Group 6 – 8 = -2 b) Br, bromine. Group 7 – 8 = -1 c) Ba, barium. Group 2 = +2 d) Ga, gallium. Group 3 = +3 Chapter 4 Homework Page 2 of 2











