ideal gas law
advertisement

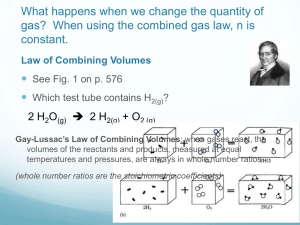
Section 13.2 Using Gas Laws to Solve Problems Learning Goal: I will be able to… … solve gas problems using the ideal gas law or any other gas law. Section 13.2 Using Gas Laws to Solve Problems A. The Ideal Gas Law • Boyle’s Law • Charles’s Law • Avogadro’s Law Combined gas law: PV/T = k PV = k (at constant T and n) V/T = b (at constant P and n) V/n = a (at constant T and P) Volume depends on T, P, and n: V = R(Tn/P) Combining and rearranging the equation gives the ideal gas law: PV = nRT universal gas constant R = 0.08206 L atm/K mol units must be P in atm, V in L, T in K Section 13.2 Using Gas Laws to Solve Problems The Ideal Gas Law • Most gases obey this equation closely / behave ideally at pressures of ~1 atm or lower, and ~0oC or higher. • You should assume ideal gas behavior when working your chemistry problems. • This equation can be used to solve all types of gas law problems: 1. List the initial and final conditions of the gas 2. If needed, convert P to atm, V to L, T to K 3. Rearrange the equation so that the quantities that change are on one side and the quantities that remain constant are on the other side; this will simplify the equation: ex.: you may end up with Boyle’s equation or Charles’ equation Section 13.2 Using Gas Laws to Solve Problems Example: Initial Conditions P1 = 0.454 atm V1 = 3.48 L T1 = 258 oK Final Conditions P2 = 0.616 atm V2 = ? T2 = 309 oK (oK = oC + 273) Notice that n is not given, but we know it has not changed. PV = nRT should be rearranged as: Change Constant PV = nR T which leads to P1V1 = nR = P2V2 and P1V1 = P2V2 T1 T2 T1 T2 Solving for V2 will give us the answer: V2 = P1V1 T2 = 3.07 L T1P2 Section 13.2 Using Gas Laws to Solve Problems C. Gas Stoichiometry (covered before) Molar Volume • Standard temperature and pressure (STP) – 0oC and 1 atm • For one mole of a gas at STP • Molar volume of an ideal gas at STP = 22.4 L • Mole-mole ratio = liter-liter ratio for gases Section 13.2 Using Gas Laws to Solve Problems B. Dalton’s Law of Partial Pressures • What happens to the pressure of a gas as we mix different gases in the container? • For a mixtures of gases in a container, the total pressure exerted is the sum of the partial pressures of the gases present. Ptotal = P1 + P2 + P3 • The pressure of the gas is affected by the number of particles • The pressure is independent of the nature of the particles Section 13.2 Using Gas Laws to Solve Problems B. Dalton’s Law of Partial Pressures Therefore: • The volume of the individual particles is not very important. • The forces among the particles must not be very important. Section 13.2 Using Gas Laws to Solve Problems B. Dalton’s Law of Partial Pressures (not in notes) Collecting a gas over water • Total pressure is the pressure of the gas + the vapor pressure of the water. Section 13.2 Using Gas Laws to Solve Problems B. Dalton’s Law of Partial Pressures (not in notes) Collecting a gas over water • How can we find the pressure of the gas collected alone? 13.3 Section 13.2 Using Gas Laws to Solve Problems B. The Kinetic Molecular Theory of Gases 13.3 Section 13.2 Using Gas Laws to Solve Problems D. Real Gases • Gases do not behave ideally under conditions of high pressure and low temperature. Why? • At high pressure the volume is decreased – Molecule volumes become important – Attractions become important (London disp. forces)