What happens when we change the quantity of constant.
advertisement
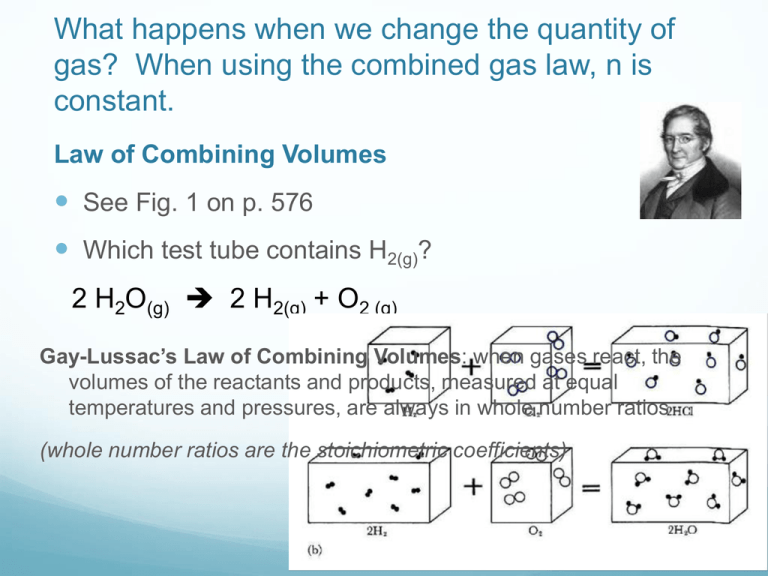
What happens when we change the quantity of gas? When using the combined gas law, n is constant. Law of Combining Volumes See Fig. 1 on p. 576 Which test tube contains H2(g)? 2 H2O(g) 2 H2(g) + O2 (g) Gay-Lussac’s Law of Combining Volumes: when gases react, the volumes of the reactants and products, measured at equal temperatures and pressures, are always in whole number ratios (whole number ratios are the stoichiometric coefficients) How can 2 samples of gas that contain particles of different mass have equal pressures at the same T? Avogadro’s Hypothesis: equal volumes of all ideal gases at the same T and P contain the same number of molecules Fig 4 p. 578 Molar Volume At STP, 1 mole of an ideal gas will have a volume of ______. At SATP, 1 mole of an ideal gas will have a volume of 24.8 L. Then how do gases differ from one another??? mass (due to different molar masses) density Ideal Gas Law R= universal gas constant R= 8.314 kPa L/mol K Derive R @ STP… R= 0.082058 atm L/ mol K R= 62.37 mmHg L/ mol K Dalton’s Law of Partial Pressures Ptotal= P1 + P2 + P3 + … +Pn Imagine mixing 3 different gases each having a different pressure… What would the final pressure be? Dalton’s Law of Partial Pressures: the total pressure of a mixture of gases is the sum of the pressures of each of the individual gases Ex. What is the pressure of O2 in the atmosphere? Practice Pg. 594 #1-4 Pg. 596 #1-3