Unit 4 – Gases
advertisement

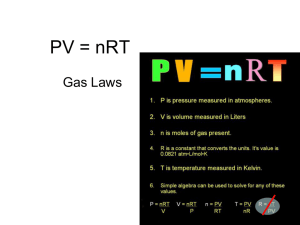
Unit 5 – Gases 11.1 - states of matter and motion: solid – vibrate, liquid – rotate, gas – translate - attractions between the particles - kinetic molecular theory of gases 11.2, 11.4, 11.5 - components of the atmosphere - greenhouse effect and global warming - air quality, smog, pollutants; AQHI - indoor air quality and factors that compromise it 11.7 - air pressure, evidence of atmospheric pressure, measuring air pressure - history: changes in atmospheric pressure and the barometer - units: 101.3 kPa = 1 atm = 760 torr = 760 mmHg - STP and SATP 11.8 - temperature, Celsius and the Kelvin (absolute) scale, absolute zero - Charles’ Law: V1/T1 = V2/T2 (T is in Kelvin), balloon 11.9 - Boyle’s Law: P1V1 = P2V2, piston - Gay-Lussac’s Law: P1/T1 = P2/T2 (T is in Kelvin), scuba tank - combined gas law: P1V1/T1 = P2V2/T2 (T is in Kelvin) 12.1 - Gay-Lussac’s Law of Combining Volumes: gases combine in small, whole number ratios - Avogadro’s Law V1/n1 = V2/n2 - Avogadro’s Law V1/n1 = V2/n2 - molar volume is 22.4 L/mol, calculate #L = n x 22.4L/mol at STP - density, D = MM/MV - Avogadro’s Hypothesis: equal volumes of gases contain an equal number of particles 12.2 - real vs. ideal gases - why a real gas deviates from an ideal gas - ideal gas law: PV = nRT, R is the universal gas constant 12.4 - Dalton’s Law of partial pressures: P1 + P2 + P3 = PTotal - collecting a gas over water: Pgas Ptotal - PH2O (Dalton Law of PP) 12.5 - stoichiometry, n may be calculated in 4 equations (12.1 and 12.2) - MM = m/n, to determine the MM of a gas