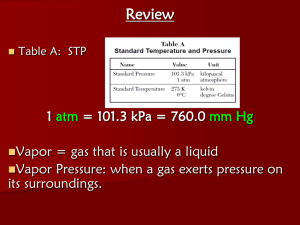
1110599Notes 14.1-14.2
advertisement
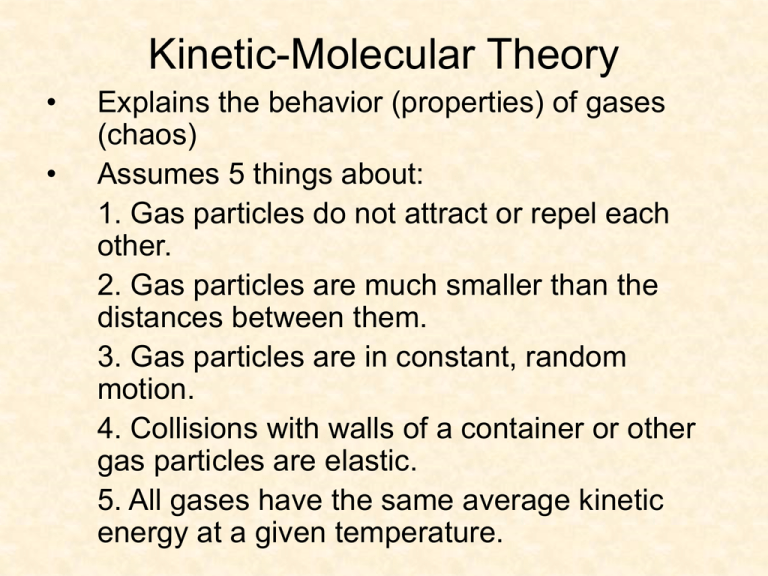
Kinetic-Molecular Theory • • Explains the behavior (properties) of gases (chaos) Assumes 5 things about: 1. Gas particles do not attract or repel each other. 2. Gas particles are much smaller than the distances between them. 3. Gas particles are in constant, random motion. 4. Collisions with walls of a container or other gas particles are elastic. 5. All gases have the same average kinetic energy at a given temperature. Ideal vs. Actual Gases • Gases don’t behave exactly the way scientists predict with the k-mt but it’s close. • Main factors to consider about a gas: 1. number of gas particles 2. volume of gas 3. temperature of gas 4. pressure of gas • These 4 factors influence each other in predictable ways. • Boyle’s Law: relationship between pressure & volume • Charles’ Law: relationship between volume & temperature • Gay-Lussac’s Law: relationship between temperature & pressure • For each of the above decide: – Inverse or direct relationship? – What would graph look like? – Formula for finding unknown variable? Try It 1. The volume of a gas at 99.0 kPa is 300.0 mL. If the pressure is increased to 188 kPa, what will be the new volume? 2. A gas at 89o C occupies a volume of .67 L. At what Celsius temperature will the volume increase to 1.12 L? 3. A gas in a sealed container has a pressure of 125 kPa at a temperature of 30.0o C. If the pressure in the container is increased to 201 kPa, what is the new temperature? Answers 1. P1V1 = P2V2 99.0 x 300.0 = 188 x V2 158 mL 2. V1/T1 = V2/T2 .67 L / 362 K = 1.12 L / T2 332 o C 3. P1/T1 = P2/V2 125 kPa / 303 K = 201 kPa / T2 487 K or 214 o C Combined Gas Law • All 3 gas laws in one. All 3 factors may vary or one may be held constant. P1V1 / T1 = P2V2 / T2 • Why aren’t weather balloons fully inflated before they are released? Try It 1. A helium-filled balloon at sea level has a volume of 2.1 L at .998 atm and 36o C. If it is released and rises to an elevation at which the pressure is .900 atm and the temperature is 28o C, what will be the new volume of the balloon? 2. At 0.00o C and 1.00 atm pressure, a sample of gas occupies 30.0 mL. If the temperature is increased to 30.0o C and the entire gas sample is transferred to a 20.0 mL container, what will be the gas pressure inside the container? Answers 1. .998 atm x 2.1 L ------------------------------------ .900 atm x V2 = 309 K V2 = 2.27 L 301 K 2. 1.00 atm x 30.0 ml ------------------------------------ 273 K P2 = 1.66 atm ---------------------------------- P2 x 20.0 ml = ---------------------------------- 303 K