Gas Law Notes
advertisement

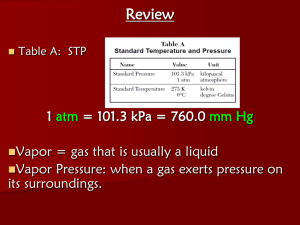
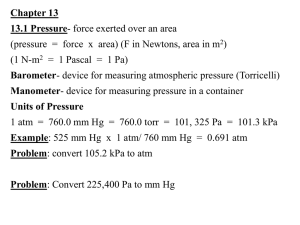
Chapter 12 Notes As students, professors, and chemists, we sometimes need to understand the concepts before we can apply it, and assuming the gases are in an ideal state where it is unaffected by real world conditions will help us better understand the behavior the gases. In order for a gas to be ideal, its behavior must follow the KineticMolecular Theory whereas the Non-Ideal Gases will deviate from this theory due to real world conditions. Kinetic Molecular Theory of gases 1. Gases consist of tiny particles – atoms or molecules 2. They are so small compared to the distance between them, that the size of the individual particles (their volume) is negligible. 3. Particles are in constant random motion, colliding with the walls of the container . These collisions with the walls cause the pressure exerted by a gas 4. Particles don’t repel or attract one another 5. Average kinetic energy is directly proportional to the Kelvin temperature. Implications 1. Temperature is temperature of a gas reflects how fast gas particles are moving a. Hi temp – fast b. Lo -slow 2. As gas moves faster, hits walls harder, so increase pressure 3. Increase temp, particles faster, increase pressure, which will increase the volume Put your finger on the constant Pressure Temperature Volume **Boyle’s Law – at a constant temperature and mass, a gas’s pressure and volume are inversely proportional to one another P1V1 = P2V2 **Charles’s Law – at constant pressure and mass, a gas’s volume and temperature are proportional to another V1 = T1 V2 T2 Amonton’s Law - given a constant number of mole of a gas and an unchanged volume, pressure is directly proportional to P1 = T1 temperature P2 T2 Avagadro’s Law - Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure V1 = n1 V2 n2 **Gay- Lussac’s Law (Combined Law) - P 1V 1 = T1 P 2V 2 T2 **Ideal Gas Law PV = nRT OR P 1V 1 = n1RT1 P 2V 2 n2RT2 **Dalton’s Law of Partial Pressures PT = P 1 + P2 + P3 + Petc…… Variables Factor Pressure Variable P Volume V Moles Temperature Gas Constant n T R Units atm Torr Pa mmHg L m³ mol K 0.0821L •atm Standard Temperature and Pressure (STP) Standard condition of temperature and pressure is known as STP. Two things you should know about this is listed below. o The universal value of STP is 1 atm (pressure) and 0 C. Note that this form specifically stated 0o C degree, not 273 Kelvin, even thought you will have to convert into Kelvin when plugging this value into the Ideal Gas equation or any of the simple gas equations. In STP, 1 mole of gas will take up 22.4 L of the volume of the container (we will use this concept next chapter) STP = 273K at 1 atm Conversions Pressure: (at sea level) 1 atm = 760.0 mm Hg = 760 torr =101.326 KPa = 101,326 Pa Volume: 1 cm3 (cubic centimeter) = 1 mL (milliliter) Temperature: o o F = (1.8oC) + 32 C = (F –32)/1.8 o o K = C + 273 C = K - 273