Chapter 2 Valence bond theory
advertisement

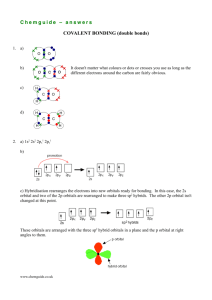
CHAPTER 2: VALENCE BOND THEORY HOMONUCLEAR DIATOMIC MOLECULES: VALENCE BOND (VB) THEORY The word homonuclear is used in two ways: • A homonuclear covalent bond is formed between atoms of the same element, e.g. the H – H bond in H2, the O = O bond in O2 and the O – O bond in H2 O2 . • A homonuclear molecule contains one type of element, e.g. H2, N2 and F2 and larger molecules such as O3, P4, S8 and C60. CHEM210/Chapter 2/2014/01 Before discussing covalent bonding, we consider the following: • For an atom X, the value of the single bond covalent radius, rcov, is half the internuclear separation in a homonuclear X – X bond. • The van der Waals radius, rv, of an atom X is half of the distance of closest approach of two non-bonded atoms of X In covalent bonding, as two nuclei approach each other their atomic orbitals overlap. • As the amount of overlap increases, the energy of the interaction decreases. • At some distance the minimum energy is reached. • The minimum energy corresponds to the bonding distance (or bond length). • As the two atoms get closer, their nuclei begin to repel and the energy increases. At the bonding distance, the attractive forces between nuclei and electrons just balance the repulsive forces (nucleus-nucleus, electron-electron). CHEM210/Chapter 2/2014/02 CHEM210/Chapter 2/2014/03 A covalent bond forms when the orbitals of two atoms overlap and the overlap region, which is between the nuclei, is occupied by a pair of electrons. A set of overlapping orbitals has a maximum of two electrons that must have opposite spins. The greater the orbital overlap, the stronger (more stable) the bond. The valence atomic orbitals in a molecule are different from those in isolated atoms. There is a hybridization of atomic orbitals to form molecular orbitals. CHEM210/Chapter 2/2014/04 CHEM210/Chapter 2/2014/05 HYBRIDIZATION The number of hybrid orbitals obtained equals the number of atomic orbitals mixed. The type of hybrid orbitals obtained varies with the types of atomic orbitals mixed. sp hybrid orbital atomic orbitals hybrid orbitals The sp hybrid orbitals in gaseous BeCl2. CHEM210/Chapter 2/2014/06 orbital box diagrams with orbital contours CHEM210/Chapter 2/2014/07 sp2 hybrid orbitals When we mix n atomic orbitals we must get n hybrid orbitals. sp2 hybrid orbitals are formed with one s and two p orbitals, therefore, there is one unhybridized p orbital remaining. The large lobes of sp2 hybrids lie in a trigonal plane. All molecules with trigonal planar electron pair geometries have sp2 orbitals on the central atom. The sp2 hybrid orbitals in BF3. CHEM210/Chapter 2/2014/08 sp3 hybrid orbitals sp3 hybrid orbitals are formed from one s and three p orbitals, therefore, there are four large lobes. Each lobe points towards the vertex of a tetrahedron. The angle between the large lobes is 109.5 The sp3 hybrid orbitals in CH4. CHEM210/Chapter 2/2014/09 The sp3 hybrid orbitals in NH3. CHEM210/Chapter 2/2014/10 The sp3 hybrid orbitals in H2O. CHEM210/Chapter 2/2014/11 Geometrical arrangements characteristic of hybrid orbital sets Atomic orbital Hybrid orbital set set Geometry Examples s, p Two sp BeF2, HgCl2 s, p, p Three sp2 BF3, SO3 s, p, p, p Four sp3 CH4, NH3, H2O, NH4+ CHEM210/Chapter 2/2014/12 Composition and orientation of orbitals CHEM210/Chapter 2/2014/13 MULTIPLE BONDS Have and -bonds. In -bonds, the electron density lies on the axis between the nuclei. All single bonds are -bonds. -Bonds: electron density lies above and below the plane of the nuclei. A double bond consists of one -bond and one bond. A triple bond has one -bond and two bonds. Often, the p-orbitals involved in -bonding come from unhybridized orbitals. CHEM210/Chapter 2/2014/14 Ethane, C2H6 both Cs are sp3 hybridized s-sp3 overlaps to σ bonds relatively even distribution of electron density over all σ bonds sp3-sp3 overlap to form a σ bond CHEM210/Chapter 2/2014/15 Ethylene, C2H4 One - and one -bond with both C atoms being sp2 hybridized. Both C atoms with trigonal planar electron pair and molecular geometries. CHEM210/Chapter 2/2014/16 overlap in one position - σ p overlap - π electron density CHEM210/Chapter 2/2014/17 Acetylene, C2H2 In acetylene: • the electron pair geometry of each C is linear,therefore, the C atoms are sp hybridized. • the sp hybrid orbitals form the C-C and C-H -bonds. • there are two unhybridized p-orbitals. • both unhybridized p-orbitals form the two -bonds, one -bond is above and below the plane of the nuclei and the other -bond is in front and behind the plane of the nuclei. When triple bonds form (e.g. N2), one bond is always above and below and the other is in front and behind the plane of the nuclei. Electron density and bond order. CHEM210/Chapter 2/2014/18 EXAMPLE Describe the types of bonds and orbitals in acetone, (CH3)2CO SOLUTION Use the Lewis structures to ascertain the arrangement of groups and shape at each central atom. Postulate the hybrid orbitals taking note of the multiple bonds and their orbital overlaps. O H H C H C O H C H H C H3 C σ bonds CH3 π bonds CHEM210/Chapter 2/2014/19 Delocalized π bonding So far all the bonds we have encountered are localized between two nuclei. In the case of benzene: • there are 6 C-C bonds, 6 C-H bonds. • each C atom is sp2 hybridized. • there are 6 unhybridized p orbitals on each C atom. CHEM210/Chapter 2/2014/20 In benzene there are two options for the 3 bonds: • localized between C atoms or • delocalized over the entire ring (i.e. the electrons are shared by all 6 C atoms). Experimentally, all C-C bonds are the same length in benzene, therefore, all CC bonds are of the same type (recall single bonds are longer than double bonds). CHEM210/Chapter 2/2014/21 Restricted rotation of π-bonded molecules in C2H2Cl2 cis- trans- CHEM210/Chapter 2/2014/22 General conclusions on multiple bonds • • • • Every two atoms share at least 2 electrons. Two electrons between atoms on the same axis as the nuclei are bonds. bonds are always localized. If two atoms share more than one pair of electrons, the second and third pair form bonds. • When resonance structures are possible, delocalization is also possible. CHEM210/Chapter 2/2014/23