Chemistry Midterm Test November 10, 2006
advertisement

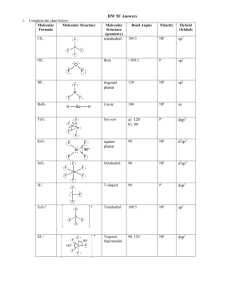
Chemistry Midterm Test November 10, 2006 Total Score: 100 points Time: 2 hours (18:00-20:00) You are not allowed to ask TAs questions regarding the content of the test. However, using non-programmable calculators is allowed. 2 1. (10%) The energy level of a particle in a box can be expressed as En = n2 h 2 8mL (n=1, 2, 3, … ) where L is the width of the box, m is the mass of the particle, h is the Planck’s constant, and n is the quantum number. (a) What is the energy released when a transition from n = 3 to n = 1 states occurs? (b) What is the wavelength of the radiation if the energy is released as photo emission? Note: You do not need to obtain numerical results. Instead, you only need to express your result in terms of the variables defined above. 2. (15%)(a) How many orbitals in the n =3 shell? (b) How many electrons can this shell host at most? (c) We can use three quantum numbers (n, l, ml) to describe an orbital. Write down the quantum numbers corresponding to a 3f2 orbital. 3. (10%) Which ion of each of the following pairs has the larger radius: (a) Ca2+, Ba2+ (b) As3-, Se24. (10%) Give the ground state electron configuration and number of unpaired electrons expected for each of the following ions: (a) Cu2+ (b) S2- (c) Ni2+ (d) I- (e) Sn4+. 5. (10%) Draw the Lewis structure and determine the formal charge on each atom in (a) CCl4 (b) NO+ (c) OCl- (d) peroxide, O22- (e) nitronium ion, ONO+. 6. (10%) For each pair, determine which compound has bonds with greater ionic character: (a) PH3 or NH3 (b) HCl or HI (c) CH4 or CF4 (d) CO2 or CS2 (e) SF6 or IF5 7. (4%) Place the species below in order of shortest to longest nitrogen-to-nitrogen (N-N) bond: N2, N2F4, and N2F2.(2%) Explain your answers.(2%) 8. (5%) Draw the σ bonding and σ∗ antibonding orbitals that correspond to the direct orbital overlap in molecules of (a) H2 and (b) HF, respectively.(2% each) Compare the difference.(1%) 9. (6%) Consider the molecule H2C = C = C= CH2 (a) Draw the overlap of p-orbitals of all three carbon atoms.(3%) (b) Based on the hybridization of each carbon atom, do all the hydrogen atoms on the same plane and why?(3%) 10. (20%) Multiple choice questions. Exactly one answer is correct. (1) What is the shape of ICl4 ? (A) T-shaped (B) trigonal bipyramidal (C) seesaw (D) tetrahedral (E) square planar (2) Which of the following only has bond angles of 90o? (A) IF5 (B) IF4+ (C) XeF2 (D) SF4 (E) IO2F3 (3) All of the following have an linear shape except (B) CS2. (C) COS. (D) I3(A) CH22+ (E) I3+ (4) All of the following are polar except (A) SF4 (B) ClO2 (C) IF4+ (E) ClF3 (D) XeF4 (5) Which of the following species has the longest bond length? (B) NO (C) N22+ (D) N22(E) O22(A) N2 (6) Which of the following is paramagnetic? (B) N22+ (C) O22(D) N22(A) N2 (E) NO+ (7) The bond order of O22+ is (A) 1 (B) 2 (C) 3 (E) 1.5 (D) 2.5 (8) Two Lewis structures can be written for diazomethane, where the arrangement of atoms is H2C-N-N. The hybrid orbitals used by the bold atoms in these Lewis structures are (B) sp2 and sp. (C) sp3 and sp. (A) sp3 or sp2, and sp. (D) sp3 or sp2, and sp2. (E) none of the above is correct. (9) Which of the following species has two unpaired electrons? (B) NO+ (C) CO+ (D) NF+ (E) CF+ (A) OF+ (10) For peroxyacetylnitrate, CH3C(=O)O—ONO2, what hybrid orbitals are used by the oxygen atom in bold? (D) sp3 (E) dsp2 (A) dsp (B) sp (C) sp2