File
advertisement

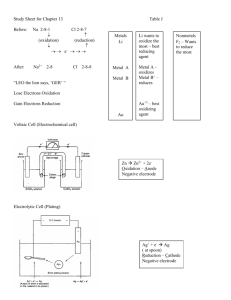
Chemistry Class Notes. 1 May 2013 CELLS In chemistry, a cell is a device in which redox reaction takes place. Electrodes are metal wire/rod/strip on which redox reaction takes place Anodes are the electrode on which oxidation takes place -negative -electrons leave here Cathodes are the electrode on which reduction takes place -positive -electrons come here 2 types of cell 1.electrochemical-where electrical energy is produced at the expense of chemical change 2.electrolytic cell- involves use of electrical energy for chemical change ELECTRODES Whenever any metal is dipped in its ionic solution there exists and maintains equilibrium between it and its ionic state. This is an electrode. Whenever you join any two electrodes together, one will gain electrons, and one will lose electrons. A.K.A. redox reactions will take place. The electrons flow –ve to +ve. The electricity flows the opposite way, +ve to –ve. In all redox reactions anodes will have an excess of –ve ions, cathodes will have an excess of positive ions. After a while the reaction will stop, the solutions will repel the electrons because of their charge. SALT BRIDGE Can only be made of KCl, KNO3, NH4NO3, usually we use KNO3, the ions and cations have a very close ionic speed. Has to be semi-solid or aqueous. The salt bridge carries the excess ions to the other side. 2 function 1. To complete circuit a. It makes a full circuit, now two bridges, aka a circle 2. To neutralize the excess charge, to maintain electrical neutrality in solution Agar-agar, means it’s semi-solid, jelly-like. 3 May 2013 Reactivity series, same as electrochemical series ELECTRO CHEMICAL CELLS Anode, negative, typically on the left Cathode, positive, right Any two metals dipped in their ionic solution connected by a wire will conduct electricity What is neutrality? Excess positive on the left hand side, in the anode Excess negative, electrons, on the right, cathode Salt can conduct electricity in Molten state, jelly Emf Emf= Electro-motive force = Eright-Eleft (Electro potential of the right – electrode potential of the left) Es given in data booklet Negative, or 0, means NO reaction will happen It has to be positive for a reaction to take place Example Iron rod in CuSO4, copper sulfate is blue Fe -> fe 3+ = 3e-, oxidizing, left Cu2+ +2e- -> Cu (s) , reducing, right Emf = 0.34 – -0.45 =0.79 V A reaction takes place ELECTROCHEMICAL SERIES From it you can predictl what will replace what Whatever is going to replace should be stronger, closer to the top of the reactivity series A stronger metal will replace a weaker metal Whatever metal is costlier wil not react, gold, platinum, they’re nice because they don’t react SPM Semi permeable membrane Only allows solvent to pass through it Water can get into an egg, but the egg part can’t get out ELECTROLYTIC CELL Opposite to electro chemical, negative on the right, positive on the right Used to purify costly metals, the one on the left is impure and it dissolves, and the right one is more pure The impure metal that dissolves is called anode mud Two copper rods in copper sulfate solution Cu -> cu2+ + 2e-, oxidation Cu 2+ 2e- -> cu , reduction Electrochemical 1. Produces electricity 2. Anode is negative, cathode is positive 3. Salt bridge needed 4. Electrolytic 1. Uses electricity 2. Anode is positive 3. No Salt bridge required NaCl: Na -> Na+ +eNa + H20 -> NaOh + H2 Don’t use aq NaCL, it makes h gas, it is inefficient, use molten