ElectroChem
advertisement

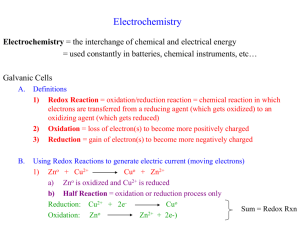
Electrochemistry Experiment 12 Oxidation – Reduction Reactions • Consider the reaction of Copper wire and AgNO3(aq) AgNO3(aq) Cu(s) Ag(s) Oxidation – Reduction Reactions • If you leave the reaction a long time the solution goes blue! • The blue is due to Cu2+(aq) Oxidation-Reduction Reactions • So when we mix Ag+(aq) with Cu(s) we get Ag(s) and Cu2+(aq) • Ag+(aq) + 1e- Ag(s) • Cu(s) Cu2+(aq) + 2e- gain electrons = reduction lose electrons = oxidation • The electrons gained by Ag+ must come from the Cu2+ • Can’t have reduction without oxidation (redox) • Each Cu can reduce 2 Ag+ 2Ag+(aq) + 2e- 2Ag(s) Cu(s) Cu2+(aq) + 2e2Ag+(aq) + 2e- + Cu(s) 2Ag(s)+ Cu2+(aq) + 2e- Redox Cu/Ag E Cu electron flow Ag+ Ag+ Redox Cu/Ag e = charge on an electron V = Voltage in a electrochemical cell E Cu2 + ΔE = e.V Ag Ag Redox Reactions & Current • redox reactions involve the transfer of electrons from one substance to another • therefore, redox reactions have the potential to generate an electric current • in order to use that current, we need to separate the place where oxidation is occurring from the place that reduction is occurring 7 Electric Current Flowing Directly Between Atoms 8 Electric Current Flowing Indirectly Between Atoms Tro, Chemistry: A Molecular Approach 9 Electrochemical Cells • electrochemistry is the study of redox reactions that produce or require an electric current • the conversion between chemical energy and electrical energy is carried out in an electrochemical cell • spontaneous redox reactions take place in a voltaic cell – aka galvanic cells • nonspontaneous redox reactions can be made to occur in an electrolytic cell by the addition of electrical energy Tro, Chemistry: A Molecular Approach 10 Electrochemical Cells • oxidation and reduction reactions kept separate – half-cells • electron flow through a wire along with ion flow through a solution constitutes an electric circuit • requires a conductive solid (metal or graphite) electrode to allow the transfer of electrons – through external circuit • ion exchange between the two halves of the system – electrolyte Tro, Chemistry: A Molecular Approach 11 Electrodes • Anode (donates electrons to the cathode) – electrode where oxidation occurs – anions attracted to it – connected to positive end of battery in electrolytic cell – loses weight in electrolytic cell • Cathode (attracts electrons from the anode) – electrode where reduction occurs – cations attracted to it – connected to negative end of battery in electrolytic cell – gains weight in electrolytic cell • electrode where plating takes place in electroplating Tro, Chemistry: A Molecular Approach 12 Voltaic Cell the salt bridge is required to complete the circuit and maintain charge balance Tro, Chemistry: A Molecular Approach 13 Current and Voltage • the number of electrons that flow through the system per second is the current – unit = Ampere – 1 A of current = 1 Coulomb of charge flowing by each second – 1 A = 6.242 x 1018 electrons/second – Electrode surface area dictates the number of electrons that can flow • the difference in potential energy between the reactants and products is the potential difference (the potential for an electric field to cause an electrical current) – unit = Volt – 1 V of force = 1 J of energy/Coulomb of charge – the voltage needed to drive electrons through the external circuit – amount of force pushing the electrons through the wire is called the electromotive force, emf Tro, Chemistry: A Molecular Approach 14 Cell Potential • the difference in potential energy between the anode the cathode in a voltaic cell is called the cell potential • the cell potential depends on the relative ease with which the oxidizing agent is reduced at the cathode and the reducing agent is oxidized at the anode • the cell potential under standard conditions is called the standard emf, E°cell – 25°C, 1 atm for gases, 1 M concentration of solution – sum of the cell potentials for the half-reactions Tro, Chemistry: A Molecular Approach 15 Standard Reduction Potential • a half-reaction with a strong tendency to occur has a large + half-cell potential • when two half-cells are connected, the electrons will flow so that the half-reaction with the stronger tendency will occur • we cannot measure the absolute tendency of a halfreaction, we can only measure it relative to another halfreaction • we select as a standard half-reaction the reduction of H+ to H2 under standard conditions, which we assign a potential difference = 0 V – standard hydrogen electrode, SHE 16 Tro, Chemistry: A Molecular Approach 17 Half-Cell Potentials • SHE reduction potential is defined to be exactly 0 V • half-reactions with a stronger tendency toward reduction than the SHE have a + value for E°red • half-reactions with a stronger tendency toward oxidation than the SHE have a + value for E°red • ΔE°cell = E°oxidation + E°reduction – E°oxidation = -E°reduction – when adding E° values for the half-cells, do not multiply the half-cell E° values, even if you need to multiply the half-reactions to balance the equation • ΔGocell=-nFΔE°cell Tro, Chemistry: A Molecular Approach 18 Electrochemical Cell Summary The potential bereactants, calculated knowing the reduction This manifests as acan ispotential related todifference the free energy Ecell , across of standard thethe reaction electrodes. according Where to -qEcell 2+ 2+, and The cell differing stability of (Zn(s), Cu (aq)), and products (Zn potentials. These becellused to when find Eoan for the reaction at the cathode, is the change potential energy of negative charge (-q) the relation in Gacell =can -nFE red amount Cu(s)), creates potential energy gradient through which the charges o ). and Eooxfrom (= - Ethe Then Eoox+ Eored passes anode toEothe cathode red cell migrate (from high energy to =low). 2+-(aq) Zn2+(aq) --> Zn(s) Zn(s) --> +Zn2e + 2e- -0.76VEox= 0.76V Cu2+(aq) + 2e- --> Cu(s) Ered=0.34V Ecell = 0.76V+0.34V = 1.1V e- Ecell=1.1 V salt bridge cathode anode Zn (s)--> Zn2+ (aq)+ 2e- Cu2+(aq)+ 2e- --> Cu(s) Tro, Chemistry: A Molecular Approach 20 Tonight • Construction of Voltaic Cells and Measurement of Cell Potentials Trial electrodes Trial electrodes 1 Cu/Zn 4 Zn/Pb 2 Cu/Pb 5 Zn/Ni 3 Cu/Ni 6 Pb/Ni • Use the corresponding 0.1 M metal sulfate of the same metal as the electrode in the half cell • Construct a salt bridge • Measure the voltage