Ionic Compound Nomenclature #1 Name: Physical Science Ionic
advertisement

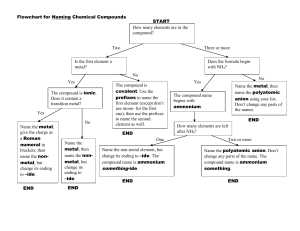
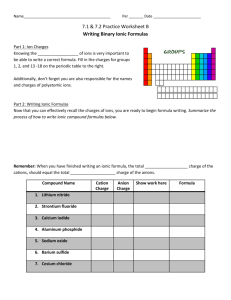
Ionic Compound Nomenclature #1 Physical Science Name: ___________________________________ Ionic Compounds: 1. write the name of the cation first 2. write the name of the anion making sure to change its name if necessary Write the name of each ionic compound. 1. KCl _______________________________________________________ 2. MgBr2 _____________________________________________________ 3. Al2S3 _____________________________________________________ 4. Na2CO3 ____________________________________________________ 5. Li3PO4 ____________________________________________________ 6. Ga(NO2)3 __________________________________________________ Write the chemical formula for each compound named. 1. write down both symbols 2. determine the charge on each one 3. charges must add up to zero so add subscripts to the elements to balance the charges 7. sodium phosphide ___________________________________________ 8. beryllium chloride ____________________________________________ 9. gallium nitride _______________________________________________ 10. magnesium nitrate ___________________________________________ 11. calcium phosphate ___________________________________________ 12. aluminum sulfate ____________________________________________ Ionic Compound Nomenclature #1 Physical Science Name: ___________________________________ Ionic Compounds with Transition Metals: 1. Write the names of the cation and the anion 2. Determine the charge of the transition metal using your charge sheet and the neutral compound rule 3. Write the charge of the transition metal using a Roman numeral in parentheses between the names of the cation & anion Write the name of each compound. 13. FeCl3 ______________________________________________________ 14. PbO ______________________________________________________ 15. Cu2S _______________________________________________________ 16. Ti (SO4)2 ____________________________________________________ 17. FePO4 ______________________________________________________ 18. Ni2S3 ______________________________________________________ Write the chemical formula for each compound. 1. Write the symbols of both ions. 2. Determine the charges, using the Roman numeral to find the transition metal’s charge 3. Add subscripts to make the charges add up to zero 19. lead (II) sulfite ______________________________________________ 20. iron (II) bromide _____________________________________________ 21. copper (I) arsenide ___________________________________________ 22. vanadium (V) phosphate ______________________________________