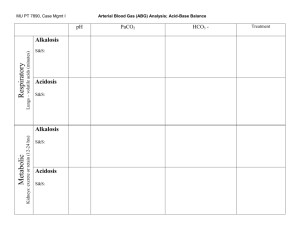
Pulmonary Physiology Equations
advertisement
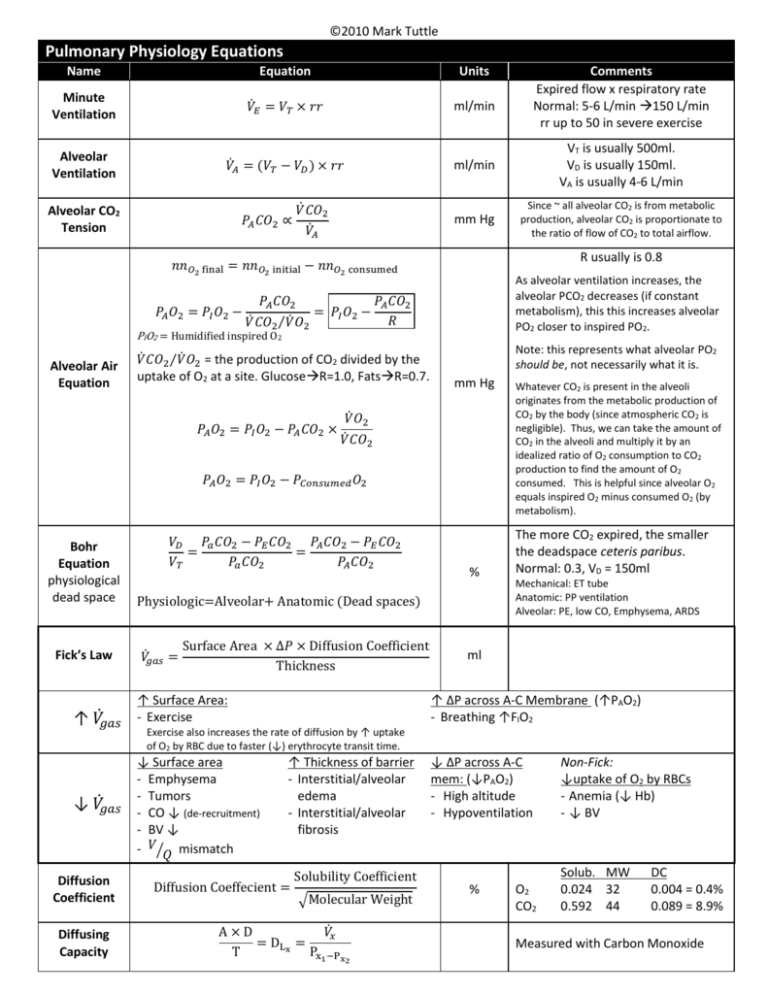
©2010 Mark Tuttle Pulmonary Physiology Equations Name Equation Units Minute Ventilation 𝑉̇𝐸 = 𝑉𝑇 × 𝑟𝑟 ml/min Alveolar Ventilation 𝑉𝐴̇ = (𝑉𝑇 − 𝑉𝐷 ) × 𝑟𝑟 ml/min VT is usually 500ml. VD is usually 150ml. VA is usually 4-6 L/min mm Hg Since ~ all alveolar CO2 is from metabolic production, alveolar CO2 is proportionate to the ratio of flow of CO2 to total airflow. Alveolar CO2 Tension 𝑃𝐴 𝐶𝑂2 ∝ 𝑉̇ 𝐶𝑂2 𝑉𝐴̇ R usually is 0.8 𝑛𝑛𝑂2 final = 𝑛𝑛𝑂2 initial − 𝑛𝑛𝑂2 consumed 𝑃𝐴 𝑂2 = 𝑃𝐼 𝑂2 − As alveolar ventilation increases, the alveolar PCO2 decreases (if constant metabolism), this this increases alveolar PO2 closer to inspired PO2. 𝑃𝐴 𝐶𝑂2 𝑃𝐴 𝐶𝑂2 = 𝑃𝐼 𝑂2 − 𝑅 𝑉̇ 𝐶𝑂2⁄𝑉̇ 𝑂2 PIO2 = Humidified inspired O2 Alveolar Air Equation 𝑉̇ 𝐶𝑂2⁄𝑉̇ 𝑂2 = the production of CO2 divided by the uptake of O2 at a site. GlucoseR=1.0, FatsR=0.7. 𝑃𝐴 𝑂2 = 𝑃𝐼 𝑂2 − 𝑃𝐴 𝐶𝑂2 × Note: this represents what alveolar PO2 should be, not necessarily what it is. mm Hg 𝑉̇ 𝑂2 𝑉̇ 𝐶𝑂2 𝑃𝐴 𝑂2 = 𝑃𝐼 𝑂2 − 𝑃𝐶𝑜𝑛𝑠𝑢𝑚𝑒𝑑 𝑂2 Bohr Equation physiological dead space Fick’s Law ̇ ↑ 𝑉𝑔𝑎𝑠 ̇ ↓ 𝑉𝑔𝑎𝑠 Diffusion Coefficient Diffusing Capacity 𝑉𝐷 𝑃𝑎 𝐶𝑂2 − 𝑃𝐸 𝐶𝑂2 𝑃𝐴 𝐶𝑂2 − 𝑃𝐸 𝐶𝑂2 = = 𝑉𝑇 𝑃𝑎 𝐶𝑂2 𝑃𝐴 𝐶𝑂2 % Physiologic=Alveolar+ Anatomic (Dead spaces) ̇ = 𝑉𝑔𝑎𝑠 Surface Area × ∆𝑃 × Diffusion Coefficient Thickness ↑ Surface Area: - Exercise Comments Expired flow x respiratory rate Normal: 5-6 L/min 150 L/min rr up to 50 in severe exercise Whatever CO2 is present in the alveoli originates from the metabolic production of CO2 by the body (since atmospheric CO2 is negligible). Thus, we can take the amount of CO2 in the alveoli and multiply it by an idealized ratio of O2 consumption to CO2 production to find the amount of O2 consumed. This is helpful since alveolar O2 equals inspired O2 minus consumed O2 (by metabolism). The more CO2 expired, the smaller the deadspace ceteris paribus. Normal: 0.3, VD = 150ml Mechanical: ET tube Anatomic: PP ventilation Alveolar: PE, low CO, Emphysema, ARDS ml ↑ ΔP across A-C Membrane (↑PAO2) - Breathing ↑FIO2 Exercise also increases the rate of diffusion by ↑ uptake of O2 by RBC due to faster (↓) erythrocyte transit time. ↓ Surface area - Emphysema - Tumors - CO ↓ (de-recruitment) - BV ↓ - 𝑉⁄𝑄 mismatch ↑ Thickness of barrier - Interstitial/alveolar edema - Interstitial/alveolar fibrosis Diffusion Coeffecient = Solubility Coefficient √Molecular Weight A×D 𝑉𝑥̇ = DL x = T Px1 −Px2 ↓ ΔP across A-C mem: (↓PAO2) - High altitude - Hypoventilation % O2 CO2 Non-Fick: ↓uptake of O2 by RBCs - Anemia (↓ Hb) - ↓ BV Solub. MW 0.024 32 0.592 44 DC 0.004 = 0.4% 0.089 = 8.9% Measured with Carbon Monoxide ©2010 Mark Tuttle Equation Units ∆𝑉 1 = ∆𝑃 𝑅 𝑜𝑓 𝑟𝑖𝑔𝑖𝑑 𝑎𝑖𝑟𝑤𝑎𝑦 L/cm H2O Name Compliance Normal: 0.2 L/cm H2O Resistance 𝑅= Poiseuille’s Equation Hemoglobin carrying capacity: 15𝑔 𝐻𝑏 𝟏. 𝟑𝟒 𝒎𝑳 𝑶𝟐 − = 20.1 𝑚𝐿 𝑂2/𝑑𝐿 100 𝑚𝐿 𝑔 𝐻𝑏 O2 Carrying Capacity of the Blood O2 Extraction 𝑉𝑂̇ 2 = 𝑄𝑡̇ (𝐶𝑎 𝑂2 − 𝐶𝑣 𝑂2 ), Turbulent Flow 𝑄𝑡̇ = mmHg L/min Law of Laplace 𝑃= 𝐶𝑎 𝑂2 − 𝐶𝑣 𝑂2 𝑉𝑂̇ 2 ρ=density of air D=diameter of airway VE=velocity of expired air η=air viscosity 2𝑇 2 × surface tension = 𝑟 radius 𝑝𝐻 = 𝑝𝑘 + 𝑙𝑜𝑔 Greater in central airways, not peripheral airways in parallel in periphery Dissolved O2 Carrying Capacity 𝟎. 𝟎𝟎𝟑 𝑚𝐿 𝑂2 𝑚𝐿 𝑂2 − 40 𝑚𝑚 𝐻𝑔 = 0.12 100 𝑚𝐿 × 𝑃𝑂2 𝑑𝐿 𝜌 × 𝑉𝐸̇ × 𝐷 𝑁𝑅 = 𝜂 Turbulent flow occurs when NR >2000 Reynold’s # HendersonHasselbalch ∆𝑃 𝑃𝑎𝑡𝑚 − 𝑃𝑎𝑙𝑣𝑒𝑜𝑙𝑎𝑟 8𝐿𝜂 𝟏 = = 4≈ 𝟒 ̇𝑉 𝑓𝑙𝑜𝑤 𝑟𝑎𝑡𝑒 𝜋𝑟 𝒓 Comments Compliance ↑: Aging + emphysema Expiration ↓ Compliance ↓: Restrictive lung diz. Inspiration ↓ L=Length of tube η=viscosity of air mmHg [𝐴− ] [𝐻𝐶𝑂3− ] = 6.1 + 𝑙𝑜𝑔 [𝐻𝐴] 0.03 ⋅ 𝑃𝐶𝑂2 - Surfactant: ↓ surface tension Deficiency: TLC ↓, ARDS/(NB),O2 tox Normal ratio: 24/1.2 = 20/1 Normal Figures Substance Normal Range Significance Blood pH 7.35 – 7.45 Indicates acidosis/alkalosis. 6.8-7.8 Compatible w/life PCO2 35-45 mmHg (40 mmHg) 1.2 mEg/L Indicates respiratory/non-respiratory cause. 40mmHg x 0.03=1.2 mEq/L [HCO3-] 22-26 mEq/L (24 mEq/L) Indicates metabolic/non-metabolic cause (7.4) Serum Anion Gap [Na+]-([HCO3-]+[Cl-]) Unmeasured ions include phosphate, citrate, sulfate, and protein. Delta Anion Gap ∆AG∆[HCO3-]=AG-1224[HCO3-] V/Q ↑ anionunmeasured to replace [HCO3-]↓in metabolic acidosis 8-16 mEq/L (12 mEq/L) If anion gap is increased, there is an increase in an unmeasured ion, usually (phosphate, lactate, β-hydroxybutyrate) If anion gap is normal in metabolic acidosis, Cl- has likely taken the place of HCO3-, called hyperchloremic metabolic acidosis. A delta-delta value below 1:1 indicates a greater fall in [HCO3-] than one would expect given the increase in the anion gap. This can be explained by a mixed metabolic acidosis, i.e a combined elevated anion gap acidosis and a normal anion gap acidosis, as might occur when lactic acidosis is superimposed on severe diarrhea. In this situation, the additional fall in HCO3- is due to further buffering of an acid that does not contribute to the anion gap. (i.e addition of HCl to the body as a result of diarrhea) A value above 2:1 indicates a lesser fall in [HCO3-] than one would expect given the change in the anion gap. This can be explained by another process that increases the [HCO3-],i.e. a concurrent metabolic alkalosis. Another situation to consider is a pre-existing high HCO3- level eg. chronic respiratory acidosis. Average: 0.8 3.3 at top, 0.65 at bottom Acid Base Disorders Primary Disturbance Acidosis Metabolic Acidosis Respiratory Acidosis Alkalosis Metabolic Alkalosis Respiratory Alkalosis Compensation from normal levels Appropriate compensation Respiratory Compensation Renal Compensation 1.2 x [HCO3-] (from 24) 0.4 x PCO2 (from 40) = PCO2 (from 40) = [HCO3-] (from 24) Respiratory Compensation Renal Copmensation 0.7 x [HCO3-] (from 24) 0.4 x PCO2 (from 40) = PCO2 (from 40) = [HCO3-] (from 24) ©2010 Mark Tuttle