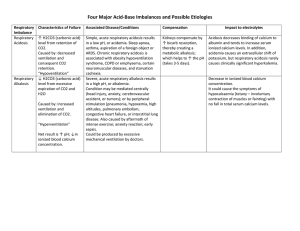
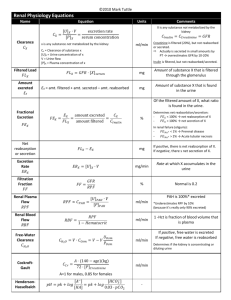
Metabolic Acidosis and Alkalosis

Metabolic Acidosis and Alkalosis
METABOLIC ACIDOSIS
ANION GAP = (Na) – (Cl) – (HCO3)
Range 8-16mmol/l
ANION GAP: MUDPILES
A KA – starved alcoholic /hi ketones/normoglycameia +/- met alk (vomiting)
M ethanol – AGA + OG, alcoholic but no ETOH, blindness
U raemia – hi Urea
D KA – BSL > 14, ketones
P araldehyde, phenformin – not seen in Aus
I ron (haematemesis, pregnancy, OD tabs on AxR) I soniazid (TB, seizures)
L actic acidosis – Lactate > 5
E thylene glycol – AGA + OG, Urine: oxalate crystals, fluoresces
S alicylates (AGA + Resp Alk, levels), starvation (ketones), strychnine
(lactate), solvent(toluene)
NB TOLUENE (glue-sniffers) can cause an elevated anion gap metabolic acidosis, and can also cause RTA (non-anion gap metabolic acidosis)
As a general rule, the diagnosis of a toxic ingestion should be actively investigated in a patient with a high anion gap acidosis where a diagnosis of ketoacidosis, lactic acidosis or renal failure is not apparent. Treatment can be life-saving if diagnosis is made early. Don't be put off if there is a normal anion gap or a normal osmolar gap as both these situations can occur even with lifethreatening ingestions.
Ketoacidosis: Can be excluded if normoglycaemia & urine negative for ketones
Lactic acidosis: Excluded if lactate level is normal. Suggested if shock or peripheral hypoperfusion.
Renal failure: Excluded as cause of acidosis if urea and creatinine normal or only slightly elevated. (In chronic renal failure acidosis is uncommon if creatinine is < 0.30 mmol/l )
Methanol: Suggested if visual impairment and CNS depression or intoxication.
Abdominal pain is common. Check the osmolar gap. Do NOT delay therapy until blood level obtained.
Ethylene glycol: Suggested if appear intoxicated and no visual disturbance.
Check the osmolar gap but it is often normal.
Salicylate: Suggested if marked hyperventilation (esp in adults) and mental obtundation.
TOXIC causes for acidosis often have PREDOMINANT NEUROLOGICAL
SIGNS
NON-ANION GAP: USED CARP (Incr Cl- as HCO3 lost)
HCO
3 loss (lower GIT, renal) rather than acid gain
Incr Cl
-
as HCO
3
lost to maintain electroneutrality
U reteral diversions
S mall bowel fistula
E xtra chloride (hi K, Urinary Na+ <10mmol/L)
D iarrhoea (low K, Urinary Na+ < 10mmol/L)
C arbonic Anhydrase Inhibitors
A drenal Insufficiency (Addisons/K+ sparing diuretics) (hi K, low Na, UrNa
>10)
R TA
P ancreatic fistula
Usually either GIT loss of base, or renal loss of base,
Diarrhoea: small bowel/whole bowel origin can cause loss of large amounts of
HCO3- and metabolic acidosis, however conditions with predominantly COLONIC pathology (U.C., Crohn’s, laxative abuse) can cause metabolic alkalosis.
METABOLIC ALKALOSIS
Caused by hi HCO3-. Severity indicated by difference between actual bicarb and expected bicarb. Kidneys RAPIDLY and EFFICIENTLY excrete HCO3- once it rises above 24mmol/l. Infusing IV bicarb will only transiently elevate serum bicarb because of prompt bicarbonaturia (if renal function normal). This is why IV bicarb can be used to alkalinise the urine.
Metabolic alkalosis therefore requires an INITIATING process and a
MAINTAINING process.
INITIATING PROCESSES
GAIN OF ALKALI IN ECF
Exogenous (IV NaHCO3 infusion, massive transfusion – citrate metabolised to
HCO3-)
Endogenous (hepatic metabolism of ketones, lactate or acetate or other anions to HCO3-)
LOSS OF H+ FROM ECF
Via Kidneys (eg diuretics)
Via Gut (vomiting, NG suction)
With normal kidneys these will cause brief/transient rise in HCO3-
MAINTAINING PROCESSES
Ie Processes that impair kidneys ability to excrete HCO3-. NB CHLORIDE
DEFICIENCY causes this because the kidney will re-absorb more HCO3- than usual to maintain electro-neutrality (as Cl- and HCO3- are the 2 main anions in the ECF, a deficiency in one leads to an increase in the other).
CHLORIDE DEPLETE – TREAT WITH NaCl
GIT Loss (vomiting, NG suction) = 90% of cases
This occurs in gastric > upper small bowel loss.
(small bowel loss > gastric loss = milder alkalosis, or if on H2 blocker with low stomach H+, may result in ACIDOSIS!)
Diuretics
Loop and Thiazides result in loss of Na+ and Cl-. Cl- loss is > HCO3- loss.
Only those who are volume deplete (hi aldosterone levels) and low dietary chloride in take are at risk. If dietary Cl- intake OK, unlikely to get alkalosis
GEORGE’s ABBREVIATED Assessment
DEPENDS ON
CLINICAL appearance
Urinary Cl-
VOLUME DEPLETED, URINARY Cl- <10mmol/l
Upper GIT loss
Diuretics
Skin loss (burns)
Treatment: NaCl (Chloride Sensitive)
VOLUME NORMAL/OVERLOADED, URINARY Cl- >10mmol/l
Hyperaldosteronism
Cushings
Treatment: Treat underlying cause (Chloride Resistant)