acid base disorders
advertisement

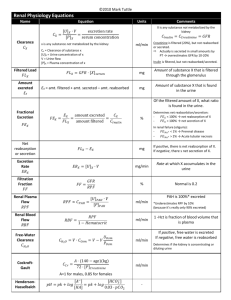
ACID BASE DISORDERS Dr. Hayam Hebah Associate professor of Internal Medicine AL Maarefa College Definitions • Acid (HA) is defined as a compound that can release a proton (H+) • Normal pH = 7.35 – 7.45 • Acidosis (acidaemia) is defined as a disorder with accumulation of acids .The pH is < 7.35 • Base (B-) can bind H+ • Alkalosis (alkalaemia) is defined as a condition with accumulation of bases . The pH is › 7.45 • Buffer is a mixture of compounds which have the ability to absorb small amounts of H+ or OH- with very little change of pH. • pH: it is a measure of H + activity Production of acids • Volatile acids: ● CO2 is a potential acid as H2CO3, and because the lungs eliminate it, it is called a volatile acid. Production of CO2 is up to 24 mol daily. ● Non-volatile acids: a) organic acids are continually produced as a by-product of metabolism: - anaerobic glycolysis in muscles → lactic acid → lactate + H+ - ketogenesis → acetoacetic acid → acetoacetate + H+ → β-hydroxybutyric acid → β-hydroxybutyrate + H+ - lipolysis → TAG → 3 FA + glycerol + 3 H+ - urea synthesis in liver: CO2 + 2 NH4 → urea + H2O + 2 H+ Under normal conditions, these acids are completely metabolized to CO2 and H2O. They have no effect on proton balance. b) inorganic acids: excretion by kidneys H2SO4 → HSO4- + H+ H3PO4 → HPO42- + H+ Note: H+ are also released from acids in the diet e. g. citric acid, ascorbic acid The body maintains ECF physiologic pH by buffers • • • • Bicarbonate buffer HCO3- / CO2 Hemoglobin (Hb) Plasma proteins (mainly albumin) Phosphate buffer HPO42- / H2PO4- (53%) (35%) (7%) (3%) NH3/NH4+ and HPO42-/H2PO4- are the most important urinary buffer systems. Mechanisms for the Homeostasis of Hydrogen Ions • Despite the continual metabolic production of acid, the pH of ECF is normally tightly maintained between 7.35-7.45. • The primary organs that deal with the acid load are: the lung (respiratory) and kidneys (metabolic). • Kidney (24-48h) The kidneys maintain the bicarbonate buffer system. They do this by retaining existing bicarbonate and generating new bicarbonate to replace that destroyed by the buffering of nonvolatile acids • Lungs ( rapid within 1-3 minutes) Eliminate carbon dioxide, maintaining a concentration of CO2 in the body fluids. ↑ ventilation → ↓ pCO2 → alkalinization ↓ ventilation → ↑ pCO2 → acidification • Chemical buffer within seconds Buffering: is the process by which a strong acid (or base) is replaced by a weaker one, with a consequent reduction in the number of free hydrogen ions. HCO3- elimination is controlled by kidneys. It takes several hours to days for urinary system to compensate for changes in pH. Liver: CO2 + 2 NH4 → urea + 2 H+ + H2O NH4+ + Glu → Gln + H2O Laboratory analysis of ABB state • Determination of pH, HCO3-, pCO2, pO2 and BE • Determination of concentration of cations (Na+, K+, Ca2+, Mg2+), concentration of anions (Cl-, lactate) and metabolites (urea, creatinine, ketone bodies) Normal values of: • HCO3- = 22 – 26 mmol/L • BE = from – 2.5 to + 2.5 mmol/L BE (base excess) is defined as the amount of acid that would be added to blood to titrate it to pH 7.4 at pCO2 = 40 mmHg. positive value = base excess negative value = base deficit (BD) Anion gap (AG) AG represents the plasma anions which are not routinely measured (albumin, phosphates, sulphates, organic anions). AG is calculated as follows: AG = (Na+ + K+) – (HCO3- + Cl-) AG= Na+ -HCO3- + Cl-) The sum of the concentrations of Na+ and K+ is greater than the sum of concentrations of HCO3- and Cl-. Difference is called as a anion gap. Normal values of AG: 16 – 20 mmol/L AG is calculated in case of metabolic acidosis. Metabolic acidosis caused by accumulation of acids in ECF. ● negative BE ● Causes: • hypoxia is a lack of O2 in tissues → anaerobic glycolysis produces lactic acid → lactate acidosis • overproduction of ketone bodies → ketoacidosis (DM, starvation) • ingestion of methanol or ethylene glycol • diarrhoea Compensation: 1st step: buffering of excess of H+ by HCO32nd step: respiratory compensation by hyperventilation 3rd step: renal correction → ↑ excretion of H+ in urine METABOLIC ACIDOSIS NORMAL ANION GAP (HYPERCHLOREMIC) 8-12 mEq HIGH ANION GAP >12 mEq • • - • Metabolic anion: -DKA -Alcoholic ketoacidosis. -Lactic acidosis -Renal failure -starvation • Drugs: -salicylates -methanol. -Ethylene glycol -carbenicillin treatment. Loss of bicarbonate: Diarrhea Pancreatic fluid loss Ileostomy Carbonic anhydrase inhibitors Chloride retention: Renal tubular acidosis. Ileal loop bladder TPN Treatment of metabolic acidosis: 1. Ttt of the cause : e.g: ttt of DKA, diarrhea, stop PF. 2-Bicarbonate if PH<7.1 3- water replacement and correction of electrolytes. 4- dialysis is necessary for severe metabolic acidosis ( as in renal failure cases) HCO3(mEq)=0.5*wt(kg)*(24-serum HCO3) Vials are 8.4%(10 mEq/10 mL). Metabolic alkalosis(MAl) • • caused by a primary accumulation of bases in ECF. the BE is increased: Both the [bicarbonate] and the [non-carbonic buffer base] are increased Causes: • ingestion of alkaline drugs (e. g. NaHCO3), MILK ALKALI SYNDROME • prolonged vomiting → loss of H+. • gastric juice loss by suction •Mineralocorticoid (renal acid excretion) •diuretic use(chloride loss in urine) . Compensation: 1st step: buffering of excess of HCO32nd step: respiratory compensation by hypoventilation → ↑ pCO2 in alveoli and arterial blood 3rd step: renal correction: ↑ excretion of HCO3- in urine treatment: • Restoration of normal body water volume, k, cl and Na • The anion given should be chloride until correction is achieved (Nacl). Respiratory acidosis (RAc) RAc is caused by hypoventilation . Hypoventilation is associated with an impaired ability to eliminate CO2, whereby pCO2 increases and the accumulated CO2 reduces the arterial pH. Causes: 1. airway obstruction: COPD , Emphysema 2. neuromuscular disorders: kyphoscoliosis, respiratory muscle weakness or paralysis 3. disorders of CNS depressing the respiratory centre :. ---opiate poisoning -----Anesthesia. Compensation: ↑ reabsorption of HCO3- is performed by kidneys (proximal tubule) Treatment: 1. Improve ventilation by maintaining open airway, use mechanical aid, bronchodilators. 2. Ttt of hypoxia by oxygen therapy 3. Endotracheal intubation 4. Noninvasive positive pressure ventilation N.B:IN cases with hypercapnia, oxygen therapy for hypoxemia may deprive the patient of the only remaining stimulus of the respiratory center and may produce more hypoventilation and co2 narcosis. Respiratory alkalosis (RAl) The hyperventilation is disproportionately high compared to the CO2 production, whereby the pCO2 falls and the pH increases Causes: 1. CNS injury 2. salicylate poisoning 3. fever, … 4. Other typical cases are the anxious patient during an attack of asthma or the hysterical hyperventilation in neurotic patients. Compensation: ↑ renal excretion of HCO3- → plasma pH decreases toward normal pH Treatment: • Reduce anxiety by anxiolytics or psychotherapy. • Tetany may be relieved by rebreathing exhaled air which will increase PCO2 and lower blood PH. Renal Tubular Acidosis • Renal tubular acidosis (RTA) is applied to a group of transport defects in the reabsorption of bicarbonate (HCO3-), the excretion of hydrogen ion (H+), or both. • The RTA syndromes characterized by: Relatively normal GFR . • Hyperchloremia Metabolic Acidosis (with normal plasma anion gap). Characteristics of types 1,2,4 RTA Type 1 Type 2 Type 4 Primary defect defect in distal Decreased proximal hydrogen ion HCO3 reabsorption excretion I e Impaired urine acidification Aldosterone def. or resistance Urine PH >5.3 Variable >5.3 if HCO3 > reaborbed threshold and < 5.3 if below Plasma HCO3 May be < 10meq/L Above 12meq/L Above 17meq/L Plasma K Usually reduced rarely elevated Normal or reduced worsen by alkali elevated ≥5.3 Type 1 Type 2 Type 4 Diagnosis Response to NaHCO3 or ammonium chloride Response to NaHCO3 Measurement of plasma aldosterone Therapeutic amount of NaHCO3 required 1-3 meq/kg/d 10-15 meq/kg/d 1-3 meq/kg/d Non-electrolyte complications Nephrocalcinosis and renal stones; Osteomalacia uncommon Rickets in children and Osteomalacia in adults; calculi is rare. None THANK YOU